Ecological Archives E096-259-A2
Jonathan S. Lefcheck and J. Emmett Duffy. 2015. Multitrophic functional diversity predicts ecosystem functioning in experimental assemblages of estuarine consumers. Ecology 96:2973–2983. http://dx.doi.org/10.1890/14-1977.1
Appendix B. A detailed description of the structural equation model and justification for included paths.
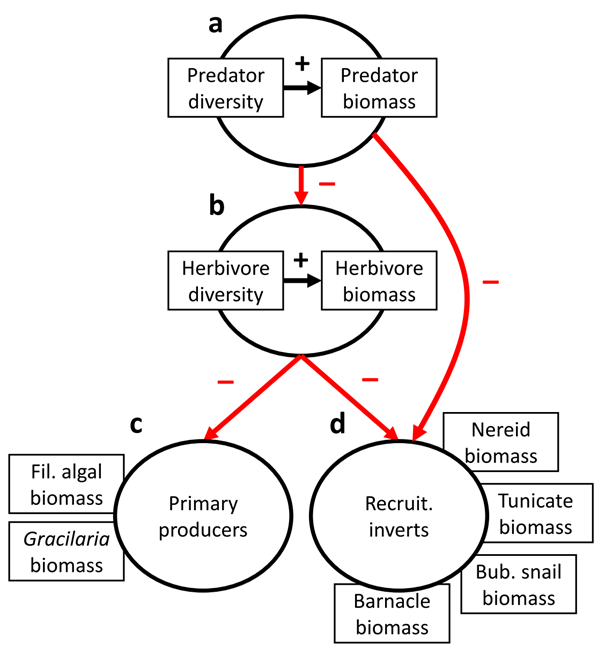
To begin, we generated a conceptual ‘meta-model’ (Fig. B1) (Grace et al. 2012). This meta-model corresponded to a simple tri-trophic food web, with predators consuming herbivores, and herbivores consuming primary producers. Both predators and herbivores were predicted to consume recruiting invertebrates, as in past experiments with these organisms (Duffy and Harvilicz 2001, Duffy et al. 2003, 2005). Within each trophic level, we had the expectation that diversity would enhance biomass (see predictions below, Fig. B1a,b). We also created composite (additive) variables to represent the entirety of final primary producer biomass, which was a combination of: final Gracilaria spp. dry mass, and recruiting filamentous algal dry mass (Fig. B1c). Similarly, recruiting invertebrate biomass was a combination of: Nereid spp. dry mass, tunicate (Mogula manhattensis) dry mass, bubble snail (Haminoea solitaria) dry mass, and barnacle (Balanus spp.) dry mass (Fig. B1d).
We populated this meta-model using variables measured during the experiment (Fig. B2). Here, we briefly describe the rationale behind each path. Letters correspond to the bubbles in Fig. B2. In all cases, ‘diversity’ can mean either functional or species richness, depending on the model considered (see Figs. 3 and 4, main text).
A) As in Fig. B1, we expected predator or herbivore diversity (functional or species richness) to enhance corresponding final biomass (Loreau et al. 2001, Duffy 2002). By including paths from both initial and final diversity to the corresponding final biomass, we can account for loss of species within replicates over the course of the experiment.
B) We also expected initial predator or herbivore diversity to predict final predator diversity. In other words, we expected to find more species left if more species were initially stocked.
C) Because we could not incorporate predators in a substitutive design, we included initial predator biomass as a covariate in all paths leading to final predator biomass. Thus, the effects of, say, final predator diversity on final predator biomass accounted for differences in initial stocked biomass within each replicate. We also included a path from initial grazer abundance to final grazer biomass for the same reason, even though we equalized grazer biomass at the beginning of the experiment (this path ended up being highly nonsignificant in all models, confirming the efficacy of our substitutive design for grazers, Table A4, A5).
D) We also included a correlation between initial predator and herbivore diversity, to account for the fact that increasing diversity necessarily meant the inclusion of more grazers and predators. This has no bearing on the model estimates, but gives an indication of how the diversity of these two trophic levels scaled as assemblages were manipulated.
E) We expected both initial and final predator biomass to decrease final grazer biomass through direct consumption. Again, by incorporating paths from both initial and final predator biomass to grazer final biomass, we can account for changes in the predator community over the course of the experiment.
F) Similarly, we expected predators change the diversity of the grazer community through the removal of (functionally distinct) species (Duffy et al. 2005, Douglass et al. 2008).
G) We expected a more diverse predator assemblage to more efficiently consume grazers by employing a diversity of foraging strategies and capture mechanisms (reviewed in Duffy et al. 2007).
H) Along similar lines, we expected a more diverse prey assemblage to enhance final predator biomass (reviewed in Duffy et al. 2007).
I) We expected final grazer biomass to decrease both final algal biomass and final recruiting invertebrate biomass via direct consumption (Duffy and Harvilicz 2001, Duffy et al. 2003).
J) We expected a more diverse predator assemblage to more efficiently consume recruiting invertebrates, for the same reasons as path G.
K) The SEMs were always a poor fit unless a direct path between final predator biomass and final algal biomass was included. This path was always positive. In light of the lack of direct negative path between final grazer biomass and primary producers (path I, Fig. B2), we interpreted this efficient consumption of grazers by predators, leading to a direct statistical effect of predators on algal resources. Had predators been less efficient or grazer biomass less depressed, we may have been able to recover paths corresponding to an indirect trophic cascade leading from predators to herbivores (negative), and herbivores to primary producers (negative).
L) Finally, we expected final grazer diversity to negatively affect final recruiting invertebrate biomass, as the invertebrates considered vary in their palatability to these small mesograzers (Duffy and Harvilicz 2001). Thus, only by including a variety of grazer species would we be able to see an effect on recruiting invertebrates as a whole.
Literature cited
Douglass, J. G., J. E. Duffy, and J. F. Bruno. 2008. Herbivore and predator diversity interactively affect ecosystem properties in an experimental marine community. Ecology Letters 11:598–608.
Duffy, J. E. 2002. Biodiversity and ecosystem function: the consumer connection. Oikos 99:201–219.
Duffy, J. E., B. J. Cardinale, K. E. France, P. B. McIntyre, E. Thébault, and M. Loreau. 2007. The functional role of biodiversity in ecosystems: incorporating trophic complexity. Ecology Letters 10:522–538.
Duffy, J. E., and A. M. Harvilicz. 2001. Species-specific impacts of grazing amphipods in an eelgrass-bed community. Marine Ecology Progress Series 223:201–211.
Duffy, J. E., J. Paul Richardson, and K. E. France. 2005. Ecosystem consequences of diversity depend on food chain length in estuarine vegetation. Ecology Letters 8:301–309.
Duffy, J. E., J. P. Richardson, and E. A. Canuel. 2003. Grazer diversity effects on ecosystem functioning in seagrass beds. Ecology Letters 6:637–645.
Grace, J. B., D. R. Schoolmaster Jr., G. R. Guntenspergen, A. M. Little, B. R. Mitchell, K. M. Miller, and E. W. Schweiger. 2012. Guidelines for a graph-theoretic implementation of structural equation modeling. Ecosphere 3:1–44.
Loreau, M., S. Naeem, P. Inchausti, J. Bengtsson, J. P. Grime, A. Hector, D. U. Hooper, M. A. Huston, D. Raffaelli, B. Schmid, D. Tilman, and D. A. Wardle. 2001. Biodiversity and ecosystem functioning: current knowledge and future challenges. Science 294:804–808.
Fig. B1. A conceptual ‘meta-model’ corresponding to a tri-trophic food web, with predators consuming herbivores, which in turn are consuming algal and invertebrate resources. Black arrows indicate hypothesized positive effects, while red arrows indicate hypothesized negative effects. Boxes surrounding circles (c) and (d) correspond to variables that were summed to create the response variable indicated in the circle that was used in the final SEM.
Fig. B2. Hypothesized causal network relating variables measured during the experiment. Expected positive relationships are given in black, and expected negative relationships are given in red.