Ecological Archives E096-102-D1
Martin M. Gossner, Nadja K. Simons, Leonhard Höck, and Wolfgang W. Weisser. 2015. Morphometric measures of Heteroptera samples in grasslands across three regions of Germany. Ecology 96:1154. http://dx.doi.org/10.1890/14-2159.1
Introduction
The consequences of environmental change (e.g., land-use intensification, climate change, habitat loss) for biodiversity and community composition have been increasingly studied by using insect traits (Vandewalle et al. 2010, Dziock et al. 2011, Börschig 2012, Moretti et al. 2013). As Díaz et al. (2013) pointed out, it is not only the species’ response to environmental change, but also the species’ functions within ecosystems which is mediated by the species’ traits. Hence, including functional traits has been highlighted as one of the most promising tools for biomonitoring (Menezes et al. 2010). The functional trait approach also provides deeper insights into community assembly (Fountain-Jones et al. 2014).
A straight-forward way to assemble traits is to use information in textbooks and other literature sources. However, this approach often only provides very generic information such as feeding mode (e.g., herbivore or carnivore) which may not be sufficient to detect the response of arthropod communities to environmental change or the consequences of trait changes for ecosystem functioning (Wright et al. 2006). It is therefore necessary to acquire additional trait information. In addition, species body size and the expression of other traits may be plastic, such that trait information gathered under a particular set of environmental conditions may not be representative of trait expression in another situation. Thus, when linking traits to species’ function in an ecosystem or to understand the response of the species community to, e.g., increased land use intensity, it is advisable to use trait information obtained under the same environmental conditions.
One method for acquiring additional trait information are morphometric measurements of species (see review by Fountain-Jones et al. 2014). Body size, for example, has been widely used as a proxy for dispersal ability, reproductive capacity, and microhabitat use (see, e.g., special feature in OIKOS; Blanchard 2011, Fountain-Jones et al. 2014). Another commonly used morphological trait is proboscis length in pollinators (Blüthgen and Klein 2011). Databases on other morphological traits are, however, rare. Individual studies on single taxonomic groups have, however, already demonstrated the value of measuring various morphological traits to analyze functional responses to disturbance, e.g., Podgaiski et al. (2013) for spiders and Ribera et al. (2001) for ground beetles.
Although European Heteroptera are a well-studied and important arthropod group in many ecosystems (Morris and Lakhani 1979, Duelli and Obrist 1998, Simons et al. 2015), a comprehensive database on morphometric traits is not yet available for this group. This is not least because systematic and taxonomic literature includes only data on particular measures that are used for species separations (see, e.g., Wagner and Weber 1964, Péricart 1972). We provide a database for 23 morphometric measures for 426 specimens of 179 species of Heteroptera, sampled by sweep-netting on a total of 150 grassland plots across three regions in Germany between 2008 and 2012. This is about 20% of all Heteroptera species recorded in Germany and more than 40% of all species described for grassland habitats in Germany.
Abstract: Trait-based approaches have increased significantly in community ecology during the last decade. This is not least because studies on biodiversity–ecosystem functioning relationships became a major topic in ecology. Species’ functions in ecosystems are mediated by their traits. For a better understanding of the relationships between environmental drivers, the community composition of organisms and ecosystems functioning, it is crucial to understand how these relationships are mediated by the communities’ trait composition. While there are world-wide efforts to set up trait databases, most have so far focused on plants and species-poorer taxa such as birds or amphibians. In contrast, for insects, the large number of species makes the gathering of comparable trait data a challenging task. In addition, there is the danger that generic trait information, which is available from common textbooks, may not be sufficient to detect the response of insect communities to environmental change or the consequences of trait changes for ecosystem functioning. One method to overcome this is to take morphometric measurements of species. In this study we measured morphometric traits of a total of 179 Heteroptera species that were sampled by sweep-netting on a total of 150 managed grassland plots across three regions in Germany between 2008 and 2012. These plots represent the whole range of grassland management intensities from extensively used pastures to mown pastures to intensively managed and fertilized meadows. In this paper we provide a database of mean values of 23 morphometric measures across sex and morphotypes for each sampled Heteroptera species. Morphological traits are assumed to be related to their adaptation and function in the environment. Thus the relative morphometric traits can be used as proxies for ecological features of a species that may affect its performance or fitness. Our database can be used by future trait-based studies for developing and testing hypotheses of the functional significance of these traits. Examples include studying the functional responses of insect communities to environmental drivers or studying how the change in trait composition affects ecosystem processes.
D. Key words: antenna length; body shape; body size; eye size; femur shape; insects; land-use intensity; leg length; rostrum length; true bugs; wing length.
Metadata
Data set descriptors
A. Data set identity: Species-level data set of 23 morphometric measures from 426 specimens of 179 species of Heteroptera.
B. Data set identification code:
1) HeteropteraMorphometricTraitsRAW.txt
2) HeteropteraMorphometricTraits.txt
C. Data set description
The data sets comprise morphometric trait data of species that were sampled and measured within the a subproject of the Biodiversity Exploratories Project (Fischer et al. 2010) which focuses on the effect of land use on arthropod community composition and related processes in three regions of Germany (Gossner et al. 2014, Simons et al. 2014, Simons et al. 2015). For details see Tables 1 and 2.
Table 1: Overview of all variables in data set 1: “HeteropteraMorphometricTraits_RAW.csv” including the 23 morphometric measurements taken in one specimen of males and females from 179 species of Heteroptera. Mean, minimum, and maximum values were calculated across all 426 measured specimens and are given in mm. The data set also includes data on order, suborder and family as well as the author name and year of species description. Sampling regions were taken from the voucher description and nearest coordinates (the center of the town for specimen from the Zoological State Collection or center of the region for specimen from the Biodiversity Exploratories and Research Collection of Martin M. Gossner) were taken from www.wikipedia.org (accessed on February, 3rd 2015).
| Variables | Type | Mean [mm] | Min [mm] | Max [mm] | Description |
ID |
integer |
|
|
|
Continuous numbering of specimens |
SpeciesID |
factor |
|
|
|
Species names |
Sex |
factor |
|
|
|
Sex of measured specimen: m=male, f=female |
Wing development |
factor |
|
|
|
Wing development of measured specimens: m=macropterous, b=brachypterous |
Source |
factor |
|
|
|
Source of specimens: Biodiversity Exploratories, Zoological State Collection Munich, Research Collection of Martin M. Gossner |
Voucher ID |
factor |
|
|
|
Detailed code of voucher |
Center_sampling_region |
coordinates |
|
|
|
Nearest coordinates of the sampling locality in WGS84 format |
Body length |
numeric |
5.00 |
1.04 |
13.90 |
From the tip of the head to the end of the abdomen |
Body width |
numeric |
1.88 |
0.41 |
8.24 |
Widest part of the body |
Body height |
numeric |
1.35 |
0.35 |
4.61 |
Thickest part of the body |
Thorax length |
numeric |
1.05 |
0.17 |
4.41 |
Longest part of the pronotum |
Thorax width |
numeric |
1.85 |
0.10 |
8.85 |
Widest part of the pronotum |
Head width |
numeric |
1.04 |
0.34 |
2.97 |
Widest part of the head including eyes |
Eye width |
numeric |
0.23 |
0.05 |
0.72 |
Widest part of the left eye |
Antenna Seg1 |
numeric |
0.54 |
0.04 |
4.13 |
Length of first antenna segment |
Antenna Seg2 |
numeric |
1.16 |
0.03 |
3.93 |
Length of second antenna segment |
Antenna Seg3 |
numeric |
0.91 |
0.14 |
3.13 |
Length of third antenna segment |
Antenna Segt4 |
numeric |
0.71 |
0.08 |
2.48 |
Length of fourth antenna segment |
Antenna Seg5 |
numeric |
1.23 |
0.50 |
2.57 |
Length of fifth antenna segment |
Front-Tibia length |
numeric |
1.23 |
0.30 |
4.10 |
Length of the tibia of the foreleg |
Mid-Tibia length |
numeric |
1.42 |
0.30 |
4.65 |
Length of the tibia of the mid leg |
Hind-Tibia length |
numeric |
2.28 |
0.13 |
8.43 |
Length of the tibia of the hind leg |
Front-Femur length |
numeric |
1.21 |
0.28 |
4.05 |
Length of the femur of the foreleg |
Hind-Femur length |
numeric |
1.81 |
0.38 |
6.70 |
Length of the femur of the hind leg |
Front-Femur width |
numeric |
0.26 |
0.06 |
0.74 |
Width of the femur of the foreleg |
Hind-Femur width |
numeric |
0.30 |
0.07 |
0.96 |
Width of the femur of the hind leg |
Rostrum length |
numeric |
2.00 |
0.42 |
7.90 |
Length of the rostrum including all segments |
Rostrum width |
numeric |
0.18 |
0.05 |
0.48 |
Widest part of the rostrum |
Wing length |
numeric |
3.80 |
0.56 |
11.85 |
Longest part of the forewing |
Wing width |
numeric |
1.19 |
0.15 |
3.85 |
Widest part of the forewing |
Table 2: Overview of all variables in data set 2: “HeteropteraMorphometricTraits.csv”. Morphometric traits were calculated from the 23 morphometric measurements measured in one specimen of males and females from 179 species of Heteroptera (see Table 1). Mean, minimum, and maximum values were calculated across all 426 measured specimens and are given in mm. The data set also includes data on order, suborder and family, the author name and year of species description as well as the number of adult specimens sampled in each region of the Biodiversity Exploratories (Schwaebische Alb, Hainich, Schorfheide-Chorin).
Trait category |
Morphometric trait |
Type |
Mean [mm] |
Min [mm] |
Max [mm] |
Description |
Body size |
Body length |
numeric |
5.11 |
1.61 |
12.96 |
Total length |
Body volume |
numeric |
30.93 |
0.39 |
409.65 |
Body length × body width × body height |
|
Dispersal ability |
Rel. Wing length |
numeric |
0.81 |
0.32 |
1.26 |
Wing length / body length |
Hind-Femur shape |
numeric |
6.33 |
2.85 |
24.71 |
Femur length / femur width |
|
Rel. Hind-Femur length |
numeric |
0.36 |
0.19 |
0.76 |
Femur length / body length |
|
Feeding resource use |
Rel. Rostrum length |
numeric |
0.41 |
0.15 |
0.69 |
Rostrum length / body length |
Front-Femur shape |
numeric |
4.93 |
2.41 |
15.84 |
Femur length / femur width |
|
Habitat use |
Body shape |
numeric |
3.11 |
1.07 |
14.16 |
Body length / body width |
Orientation |
Rel. Eye size |
numeric |
0.23 |
0.12 |
0.35 |
Eye width / head width incl. eyes |
Rel. Antenna length |
numeric |
0.68 |
0.26 |
1.44 |
Total antenna length /body length |
Overall project description
Identity: Understanding the consequences of land use in grasslands on trait composition of arthropods.
Originators: Martin M. Gossner, Nadja K. Simons, Wolfgang W. Weisser; Terrestrial Ecology Research Group, Department for Ecology and Ecosystem Management, Center for Life and Food Sciences Weihenstephan, Technische Universität München, Hans-Carl-von-Carlowitz-Platz 2, D-85354 Freising, Germany
Period of study: 2008 – 2012
Objectives: 1. To identify important traits across taxonomic groups, 2. To detect traits that respond to land-use intensification. 3. To analyze the consequences of these trait changes for ecosystem functioning.
Sources of funding: The work has been funded by the DFG Priority Program 1374 "Infrastructure-Biodiversity-Exploratories" (DFG-WE 3081/21-1.).
Site description:
Heteroptera species were sampled on 150 grassland plots within the three study regions of the Biodiversity Exploratory project: (1) the UNESCO Biosphere Reserve Schorfheide-Chorin in the North-East (53°02' N 13°83' E, about 1300 km² in size, 3–140 m a.s.l.), (2) the National Park Hainich and its surrounding areas in Central Germany (51°20' N 10°41' E, about 1300 km², 285–550 m a.s.l.), and (3) the UNESCO Biosphere Reserve Schwäbische Alb in the Swabian Jura in the South-West (48°43' N 9°37' E, about 422 km², 460–860 m a.s.l.). The grasslands are continuously managed by farmers by mowing, and/or grazing and/or fertilization. The overall management intensity covers the typical range of management intensity of the respective region (Fischer et al. 2010, Blüthgen et al. 2012).
Experimental design: Trait collection from all 179 species of Heteroptera that were sampled by sweep netting on a total of 150 grassland plots across three regions in Germany between 2008 and 2012. In June and August all plots were sampled in all years, in the months from May to October samples were taken only on a subset of plots. On each plot and during each sampling three transects of 50m were sampled with 20 double sweeps each. Samples were transferred to 70% ethanol in the field. A round sweep net with 30 cm diameter was used. In the laboratory species were sorted to taxonomic groups and all Heteroptera were identified to species level. Trait data were measured by using a high-resolution measuring stereo microscope (Leica M205 C) and the software Leica Application Suite 4 (© 2012 Leica Microsystems (Switzerland) Ltd.).
Methods
Trait collection
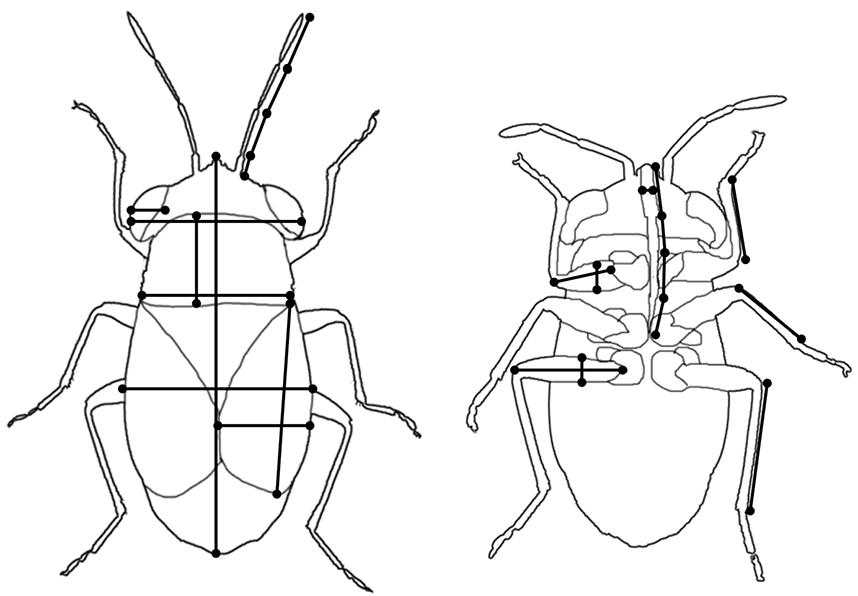
We took a total of 23 morphometric measures on each individual (see Fig. 1, Table 1) using a high-resolution measuring stereo microscope (Leica M205 C) with an apochromatic corrected 20.5:1 Zoom and the software Leica Application Suite 4 (© 2012 Leica Microsystems (Switzerland) Ltd.). We used the live-modus to measure traits by marking both ends at maximum magnification on a 20 inch display. Measured values were directly stored in an electronic data sheet.
Fig. 1. Illustration of morphometric measurements taken on each specimen from above (left) and below (right).
For all sampled Heteroptera species, morphometric traits were measured on one male and one female individual. In polymorphic species one individual of each morph across sexes was measured. Undamaged individuals of each morph and sex were selected from our samples, glued on “to glue plates” and pinned. This enabled insect bodies to be plane and they then could be aligned parallel to the lens. If necessary, specimens were detached from the “to glue plates”, e.g., for measuring rostrum length. For antenna, legs and rostrum (if necessary because of curved shape), segments were measured separately to ensure reliable measures and measured values were summed for analyses of total lengths. If no undamaged specimen from our samples was available, specimens from the Heteroptera collection of the Zoological State Collection in Munich were measured (preferably specimens sampled in Germany in similar habitats were used).
Trait classification
From the 23 morphometric measurements we derived ten morphometric traits representing five trait categories: (1) Body size was represented by the absolute body length (measured from the end of the abdomen to the tip of the head) and by body volume (calculated from body length, width and thickness following (Siemann et al. 1999). Width and thickness were measured at the widest and thickest parts, respectively, of the abdomen, thorax or head. (2) Dispersal ability was represented by relative wing length (relative to body length), by the shape of the hind femur which indicates jumping ability (femur length divided by femur width) and the relative length of the hind femur (relative to body length) which indicates running ability. The importance of increased walking speed due to longer legs and jumping capability due to thicker hind femur for colonization of habitats have already been shown for beetles and leafhoppers (Burrows and Sutton 2008; Barton et al. 2011b). High jumping ability is also described for single Heteroptera species with thick hind femur such as Halticus apterus (Linnaeus, 1758) (Wachmann et al. 2004). (3) Feeding resource use was represented by relative rostrum length (relative to body length) and by the shape of the front femur (femur length divided by femur width). In most species of Heteroptera, stylets are not much longer than the rostrum (Richards and Davies 1977) and thus we expect rostrum length to be a suitable proxy. It has been shown that switching feeding behavior requires adaptation of mouthparts in Heteroptera (Cobben 1979, Cohen 1996, Roitberg et al. 2005) and thus we expect rostrum length to depend on feeding behavior. A thick spiny front femur has been proposed to be an adaptation to seed feeding (Sweet 1963). (4) Habitat use was represented by body shape (body length divided by body width). We expect a long and thin body shape to be an adaptation to grasses. (5) Orientation ability was represented by sensory traits, i.e., relative antenna length (relative to body length) and by relative eye width (relative to head width). Orientation ability is predicted to increase with antenna length in more dense, homogeneous habitats (Barton et al. 2011) and with eye size in more open habitats (Bauer et al. 1998, Talarico et al. 2007). For each species, the mean morphometric trait value was calculated from all measured specimens.
Taxonomy and systematics:
Taxonomy follows the Fauna Europaea data base (de Jong 2013).
Acknowledgments
We thank Steffen Both, Markus Lange, Norbert Leber, Esther Pašalić, Ellen Speer, Louis Sikora, and Manfred Türke for their help with sweep-netting the grasslands, Franz Schmolke for identifying Heteroptera species, and Klaus Schönitzer from the Zoological state collection in Munich for providing specimens. We thank the managers of the three Exploratories, Kirsten Reichel-Jung, Swen Renner, Katrin Hartwich, Sonja Gockel, Kerstin Wiesner, and Martin Gorke for their work in maintaining the plot and project infrastructure; Christiane Fischer and Simone Pfeiffer for giving support through the central office, Michael Owonibi for managing the central data base, and Markus Fischer, Eduard Linsenmair, Dominik Hessenmöller, Jens Nieschulze, Daniel Prati, Ingo Schöning, François Buscot, Ernst-Detlef Schulze, Wolfgang W. Weisser and the late Elisabeth Kalko for their role in setting up the Biodiversity Exploratories project. Fieldwork permits were issued by the responsible state environmental offices of Baden-Württemberg, Thüringen, and Brandenburg (according to § 72 BbgNatSchG).
Literature cited
Barton, P. S., H. Gibb, A. D. Manning, D. B. Lindenmayer, and S. A. Cunningham. 2011. Morphological traits as predictors of diet and microhabitat use in a diverse beetle assemblage. Biological Journal of the Linnean Society 102:301–310.
Bauer, T., K. Desender, T. Morwinsky, and O. Betz. 1998. Eye morphology reflects habitat demands in three closely related ground beetle species (Coleoptera: Carabidae). Journal of Zoology 245:467–472.
Blanchard, J. L. 2011. Body size and ecosystem dynamics: an introduction. Oikos 120:481–482.
Blüthgen, N., C. F. Dormann, D. Prati, V. H. Klaus, T. Kleinebecker, N. Hölzel, F. Alt, S. Boch, S. Gockel, A. Hemp, J. Müller, J. Nieschulze, S. C. Renner, I. Schöning, U. Schumacher, S. A. Socher, K. Wells, K. Birkhofer, F. Buscot, Y. Oelmann, C. Rothenwöhrer, C. Scherber, T. Tscharntke, C. N. Weiner, M. Fischer, E. K. V. Kalko, K. E. Linsenmair, E.-D. Schulze, and W. W. Weisser. 2012. A quantitative index of land-use intensity in grasslands: Integrating mowing, grazing and fertilization. Basic and Applied Ecology 13:207–220.
Blüthgen, N., and A. M. Klein. 2011. Functional complementarity and specialisation: The role of biodiversity in plant-pollinator interactions. Basic and Applied Ecology 12:282–291.
Börschig, C. 2012. Effects of land-use intensity in grasslands on diversity, life-history traits and multitrophic interactions. Dissertation. Georg-August-Universität Göttingen.
Cobben, R. 1979. On the original feeding habits of the Hemiptera (Insecta): a reply to Merrill Sweet. Annals of the Entomological Society of America 72:711–715.
Cohen, A. C. 1996. Plant feeding by predatory Heteroptera: evolutionary and adaptational aspects of trophic switching. Zoophytophagous Heteroptera: implications for life history and integrated pest management:1–17.
de Jong, Y. S. D. M. e. 2013. Fauna Europaea version 2.6. Web Service available online at http://www.faunaeur.org.
Díaz, S., A. Purvis, J. H. C. Cornelissen, G. M. Mace, M. J. Donoghue, R. M. Ewers, P. Jordano, and W. D. Pearse. 2013. Functional traits, the phylogeny of function, and ecosystem service vulnerability. Ecology and Evolution 3:2958–2975.
Duelli, P., and M. K. Obrist. 1998. In search of the best correlates for local organismal biodiversity in cultivated areas. Biodiversity and Conservation 7:297–309.
Dziock, F., M. Gerisch, M. Siegert, I. Hering, M. Scholz, and R. Ernst. 2011. Reproducing or dispersing? Using trait based habitat templet models to analyse Orthoptera response to flooding and land use. Agriculture Ecosystems & Environment 145:85–94.
Fischer, M., O. Bossdorf, S. Gockel, F. Hansel, A. Hemp, D. Hessenmoller, G. Korte, J. Nieschulze, S. Pfeiffer, D. Prati, S. Renner, I. Schoning, U. Schumacher, K. Wells, F. Buscot, E. K. V. Kalko, K. E. Linsenmair, E. D. Schulze, and W. W. Weisser. 2010. Implementing large-scale and long-term functional biodiversity research: The Biodiversity Exploratories. Basic and Applied Ecology 11:473–485.
Fountain-Jones, N. M., S. C. Baker, and G. J. Jordan. 2014. Moving beyond the guild concept: developing a practical functional trait framework for terrestrial beetles. Ecological Entomology: doi:10.1111/een.12158.
Gossner, M. M., W. W. Weisser, and S. T. Meyer. 2014. Invertebrate herbivory decreases along a gradient of increasing land-use intensity in German grasslands. Basic and Applied Ecology 15:347–352.
Menezes, S., D. J. Baird, and A. M. V. M. Soares. 2010. Beyond taxonomy: a review of macroinvertebrate trait-based community descriptors as tools for freshwater biomonitoring. Journal of Applied Ecology 47:711–719.
Moretti, M., F. de Bello, S. Ibanez, S. Fontana, G. B. Pezzatti, F. Dziock, C. Rixen, and S. Lavorel. 2013. Linking traits between plants and invertebrate herbivores to track functional effects of land-use changes. Journal of Vegetation Science 24:949–962.
Morris, M., and K. Lakhani. 1979. Responses of grassland invertebrates to management by cutting.1. Species-Diversity of Hemiptera. Journal of Applied Ecology 16:77–98.
Péricart, J. 1972. Hémipteres Anthocoridae, Cimicidae et Microphysidae de l'Quest Palearctique. Masson et Cie Éditeurs, Paris, France.
Podgaiski, L. R., F. Joner, S. Lavorel, M. Moretti, S. Ibanez, M. d. S. Mendonça, Jr., and V. D. Pillar. 2013. Spider Trait Assembly Patterns and Resilience under Fire-Induced Vegetation Change in South Brazilian Grasslands. PLoS ONE 8:e60207.
Ribera, I., S. Doledec, I. S. Downie, and G. N. Foster. 2001. Effect of land disturbance and stress on species traits of ground beetle assemblages. Ecology 82:1112–1129.
Richards, O., and R. Davies. 1977. Imms’ general textbook of entomology. Tenth Edition, vol 1. Structure, physiology and development. Chapman and Hall, London, UK.
Roitberg, B., D. Gillespie, D. J. Quiring, C. Alma, W. Jenner, J. Perry, J. Peterson, M. Salomon, and S. VanLaerhoven. 2005. The cost of being an omnivore: mandible wear from plant feeding in a true bug. Naturwissenschaften 92:431–434.
Siemann, E., D. Tilman, and J. Haarstad. 1999. Abundance, diversity and body size: patterns from a grassland arthropod community. Journal of Anímal Ecology 68:824–835.
Simons, N. K., M. M. Gossner, T. M. Lewinsohn, S. Boch, M. Lange, J. Müller, E. Pašalić, S. A. Socher, M. Türke, M. Fischer, and W. W. Weisser. 2014. Resource-Mediated Indirect Effects of Grassland Management on Arthropod Diversity. PLoS ONE 9:e107033.
Simons, N. K., M. M. Gossner, T. M. Lewinsohn, M. Lange, M. Türke, and W. W. Weisser. 2015. Effects of land-use intensity on arthropod species abundance distributions in grasslands. Journal of Animal Ecology 48:143–154.
Sweet, M. H. 1963. The biology and ecology of the Rhyparochrominae of New England (Heteroptera: Lygaeidae). University of Connecticut.
Talarico, F., M. Romeo, A. Massolo, P. Brandmayr, and T. Zetto. 2007. Morphometry and eye morphology in three species of Carabus (Coleoptera: Carabidae) in relation to habitat demands. Journal of Zoological Systematics and Evolutionary Research 45:33–38.
Vandewalle, M., F. de Bello, M. P. Berg, T. Bolger, S. Doledec, F. Dubs, C. K. Feld, R. Harrington, P. A. Harrison, S. Lavorel, P. M. da Silva, M. Moretti, J. Niemela, P. Santos, T. Sattler, J. P. Sousa, M. T. Sykes, A. J. Vanbergen, and B. A. Woodcock. 2010. Functional traits as indicators of biodiversity response to land use changes across ecosystems and organisms. Biodiversity and Conservation 19:2921–2947.
Wachmann, E., A. Melber, and J. Deckert. 2004. Wanzen Band 2. Goecke & Evers, Keltern.
Wagner, E., and H. H. Weber. 1964. Hétéroptères Miridae. Fédération Française des Sociétés de Sciences Naturelles, Paris, France.
Wright, J. P., S. Naeem, A. Hector, C. Lehman, P. B. Reich, B. Schmid, and D. Tilman. 2006. Conventional functional classification schemes underestimate the relationship with ecosystem functioning. Ecology Letters 9:111–120.