Ecological Archives E096-059-D1
Douglas C. Woodhams, Ross A. Alford, Rachael E. Antwis, Holly Archer, Matthew H. Becker, Lisa K. Belden, Sara C. Bell, Molly Bletz, Joshua H. Daskin, Leyla R. Davis, Sandra V. Flechas, Antje Lauer, Antonio Gonzalez, Reid N. Harris, Whitney M. Holden, Myra C. Hughey, Roberto Ibáñez, Rob Knight, Jordan Kueneman, Falitiana Rabemananjara, Laura K. Reinert, Louise A. Rollins-Smith, Franklin Roman-Rodriguez, Stephanie D. Shaw, Jenifer B. Walke, and Valerie McKenzie . 2015. Antifungal isolates database of amphibian skin-associated bacteria and function against emerging fungal pathogens. Ecology 96:595. http://dx.doi.org/10.1890/14-1837.1
Metadata
Class I. Data set descriptors
Data files
Amphibian-skin_bacteria_16S_sequences.fna
Amphibian-skin_bacteria_metadata.txt
Abstract: Microbial symbionts of vertebrate skin have an important function in defense of the host against pathogens. In particular, the emerging chytrid fungus Batrachochytrium dendrobatidis, causes widespread disease in amphibians but can be inhibited via secondary metabolites produced by many different skin-associated bacteria. Similarly, the fungal pathogens of terrestrial salamander eggs Mariannaea elegans and Rhizomucor variabilis are also inhibited by a variety of skin-associated bacteria. Indeed, probiotic therapy against fungal diseases is a recent approach in conservation medicine with growing experimental support. We present a comprehensive Antifungal Isolates Database of amphibian skin-associated bacteria that have been cultured, isolated, and tested for antifungal properties. At the start, this database includes nearly 2000 cultured bacterial isolates from 37 amphibian host species across 18 studies on five continents: Africa, Oceania, Europe, and North and South America. As the research community gathers information on additional isolates, the database will be updated periodically. The resulting database can serve as a conservation tool for amphibians and other organisms, and provides empirical data for comparative and bioinformatic studies. The database consists of a FASTA file containing 16S rRNA gene sequences of the bacterial isolates, and a metadata file containing information on the host species, life-stage, geographic region, and antifungal capacity and taxonomic identity of the isolate.
D. Key words: amphibian; antifungal; Batrachochytrium dendrobatidis; culture database; disease ecology; microbiota; probiotic therapy; skin pathogens.
Class II. Research origin descriptors
A. Overall project description
A.1. Identity
This data set was compiled as part of the project entitled “Antifungal Isolates Database.”
A.2. Originators
The project was coordinated by Douglas C. Woodhams at the University of Colorado, Boulder, and currently at the University of Massachusetts Boston, Boston, MA, USA, [email protected].
A.3. Period of study
The collation of sequence data from cultured bacterial symbionts of amphibians started in 2014, but includes data from published and unpublished studies dating from 2006.
A.4. Objectives
The main objective of the project is to create a database of skin-associated bacteria that have been cultured from amphibians and tested for antifungal properties. The data will be made available to the scientific community for further analyses and conservation management approaches.
A.5. Abstract
Microbial symbionts of vertebrate skin have an important function in defense of the host against pathogens. In particular, the emerging chytrid fungus Batrachochytrium dendrobatidis, causes widespread disease in amphibians but can be inhibited via secondary metabolites produced by many different skin-associated bacteria. Similarly, the fungal pathogens of terrestrial salamander eggs, Mariannaea elegans and Rhizomucor variabilis, are also inhibited by a variety of skin-associated bacteria. Indeed, probiotic therapy against fungal diseases is a recent approach in conservation medicine with growing experimental support. We present a comprehensive Antifungal Isolates Database of amphibian skin-associated bacteria that have been cultured, isolated and tested for antifungal properties. At the start, this database includes nearly 2000 cultured bacterial isolates from 37 amphibian host species across 18 studies on five continents: Africa, Oceania, Europe, and North and South America. As the research community gathers information on additional isolates, the database will be updated periodically. The resulting database can serve as a conservation tool for amphibians and other organisms, and provides empirical data for comparative and bioinformatic studies. The database consists of a FASTA file containing 16S rRNA gene sequences of the bacterial isolates, and a metadata file containing information on the host species, life-stage, geographic region, and antifungal capacity and taxonomic identity of the isolate.
A.6. Sources of funding
The creation of the Antifungal Isolates Database was funded by the National Science Foundation (NSF; Population and Community Ecology Section DEB: 1146284 to V. McKenzie and R. Knight). Many additional funding sources supported the collection and production of the bacterial isolate data presented here, including: NSF IOS-0520847, IOS-0619536, IOS-0843207, and IOS-1121758 to LRS; DEB-0640373 to RNH; Swiss National Science Foundation 31-
125099 to DCW; ARC Discovery Grants DP0986537 and DP130101635 to RA and a US Postgraduate Fulbright Grant to JHD; Virginia Tech Department of Biological Sciences and the Fralin Life Sciences Institute to LKB; BBSRC PhD studentship to REA; NSF Graduate Research Fellowship to W. M. Holden; AZA Conservation Endowment Fund 08-836 to SVF; Auckland Zoo Charitable Trust Fund awarded to SDS.
B. Research motivation
Amphibian populations and species are increasingly threatened by emerging infectious diseases including chytridiomycosis (Skerratt et al. 2007, Fisher et al. 2012). Mitigation of disease in the field is a conservation challenge (Woodhams et al. 2011), however, probiotic strategies have been proposed as one promising avenue linking skin microbiota to pathogen tolerance (Harris et al. 2009, Bletz et al. 2013, Sutherland et al. 2014). As next-generation sequencing of microbial communities is beginning to clearly demonstrate, one of the main components defining variation in the skin microbiota is host species (McKenzie et al. 2012, Kueneman et al. 2013). While the probiotic bacterium Janthinobacterium lividum was successfully used to treat disease in trials on mountain yellow-legged frogs, Rana muscosa (Harris et al. 2009), the same probiotic treatment was not effective on another host species (e.g., Becker et al. 2011). Differences in host physiology, host immune function, or environmental conditions (e.g., temperature, Daskin et al. 2014, Woodhams et al. 2014) may constrain the antifungal bacterial communities found on amphibians. We therefore developed the Antifungal Isolate Database to centralize information available on the antifungal capacity of amphibian skin-associated bacterial cultures across geographic regions and host species.
Among other applications, the database will be used to inform selection of probiotics for conservation applications, to filter culture-independent bacterial community sequencing datasets for predicted antifungal function, and to target isolates for whole genome sequencing for identification of genes involved in antifungal capacities. Ecosystem disruption and threats to wildlife by fungal diseases are increasing (Anderson et al. 2004, Fisher et al. 2012). Thus, future enhancements of this Antifungal Isolates Database may find applications in studies of Phytophthora species and other fungal disease agents of plants (Kamoun et al. 2014), Pseudogymnoascus destructans in bats known to be affected by bacterially produced volatile organic compounds (Cornelison et al. 2014), oomycetes such as Aphanomyces astaci, Aphanomyces invadans and Saprolegnia species affecting fish and crayfish as well as amphibians (Phillips et al. 2008, Krugner-Higby et al. 2010, Van den Berg et al. 2013, Liu et al. 2014). Fusarium solani is another fungal agent of disease and mass mortality in eggs of endangered loggerhead sea turtles, Caretta caretta (Sarmiento-Ramirez et al. 2010), and at least four Fusarium spp., including F. solani, have been recognized to have a secondary role in a fungal dermatitis that caused the death of captive Wyoming toads, Anaxyrus baxteri (Perpiñán et al. 2010). With continuing improvements, this database will serve as a general resource on antifungal bacteria.
C. General methodology
C.1. Culture collection and testing for antifungal function
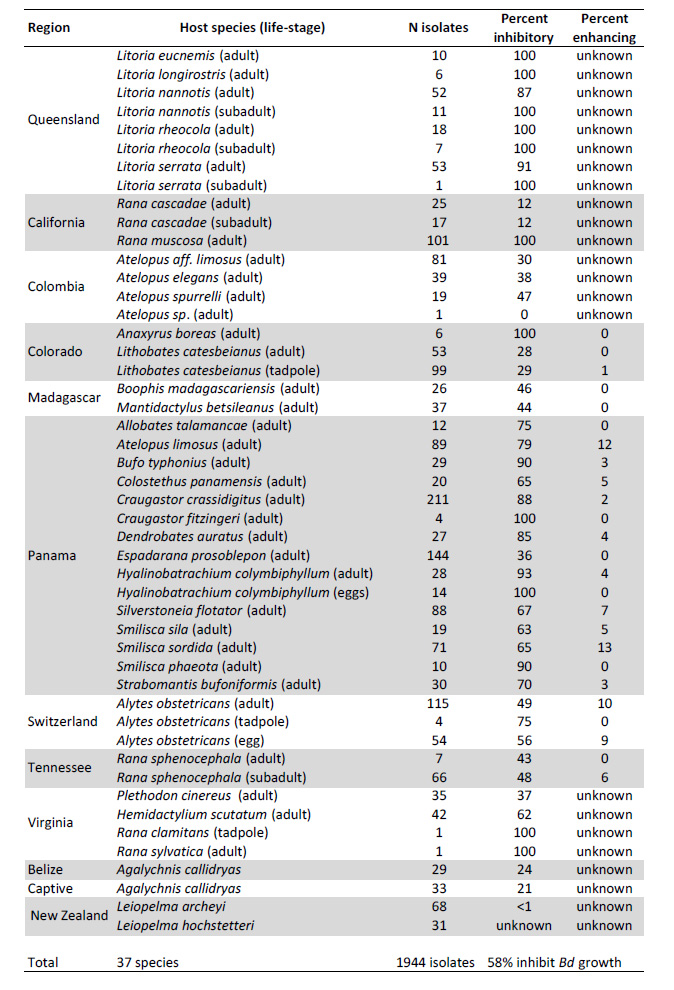
Table 1 indicates the numbers of isolates tested from each amphibian host species, life-history stage, and geographic region. Cultures were originally collected from several different studies indicated in Table 2, including a brief description of the methods used in each study. Overall, there were two types of assays used to determine the capacity of an isolate to inhibit growth of Bd: (1) Co-culture assays utilized a lawn of Bd grown on a Petri plate and a challenging isolate that was streaked across one side of the plate. If a zone of inhibition with reduced growth of Bd was observed, the isolate was considered inhibitory (Harris et al. 2006). Semi-quantitative methods have been developed for this approach (Flechas et al. 2012). (2) In a second approach, growth of Bd in liquid culture was tested upon challenge with the cell-free supernatant (filtrate) from bacterial cultures in 96-well plates. There are several variations of this approach and the response can be quantified by comparison to positive (no filtrate added) and negative (dead Bd) controls (Bell et al. 2013, Holden 2014). We did not include semi-quantitative growth inhibition of Bd in this database, but for consistency chose to indicate if an isolate was significantly inhibitory or enhancing of Bd growth based on a per-study FDR-corrected p-value of less than 0.05.
Table 1. All isolates included in the dataset. Note that some studies only sequenced inhibitory isolates (Percent inhibitory = 100%, see text section D.1), so these are over-represented in the dataset. Not all studies designated enhancing isolates.
C.2. Sequencing 16S rRNA
In each study, DNA was extracted from cultures and the 16S rRNA region amplified for Sanger sequencing. The primers varied among studies as indicated in Table 2, and the Primer sequences are given in Table 3.
Table 2. Studies included in this database are indicated by region. Study number is indicated in the metadata file for each isolate. Methods for testing antifungal function of bacterial isolates were either by examining plates with a fungal lawn for a zone of inhibition surrounding a bacterial streak, or by measuring the growth of Bd with cell-free bacterial supernatant. If not a visual response, statistical interpretation of Bd inhibition or enhancement was based on slope of Bd growth with bacterial metabolites compared to a Bd only control, or endpoint growth compared to a positive control. Because temperature can influence metabolite production (Woodhams et al. 2014, Daskin et al. 2014), temperature is indicated; room temperature was approximately 21–23°C.
Table 3. Primers used to sequence the 16S rRNA gene from bacterial isolates.
C.3. Taxonomic identification
Included in the metadata file are two columns indicating the taxonomy of the bacterial isolates. These were produced using the assign_taxonomy.py command in Qiime v.1.8.0 (Caporaso et al. 2010) using uclust with the Greengenes 13_8 reference taxonomy or the RDP_2.3 classifier program (Wang et al. 2007). Additional columns indicate the quality score for the assignment provided by the classifiers as a confidence value between 0 and 1.
D. Data limitations and potential enhancements
D.1. Bias towards antifungal isolate identification
All studies examining amphibian skin bacteria have successfully identified some antifungal isolates. However, many of the initial studies sequenced only the antifungal isolates (Table 2 studies 1,3,7,8,12). Thus, these are over-represented in the database. In other studies, all isolates were sequenced regardless of antifungal function. Two studies (Table 2 studies 13 & 14) tested isolates against fungi other than Bd. The comparative results appear in Lauer et al. (2007, 2008).
D.2. Symbiotic fungi not included
There are no published reports of fungal isolates from amphibian skin tested against Bd, although in principle, identical methodology could be employed to measure the effects of symbiotic fungi that may compete with Bd. Future versions of this database can be expanded to include fungal ITS sequences and inhibitory properties.
D3. Lack of archiving
There is currently no archiving procedure or repository for this data set other than the current publication. Sequences from some isolates included here (Lauer et al. 2008, Bell 2012, Küng et al. 2014, Woodhams et al. 2014, Daskin et al. 2014; Shaw et al. 2014; Davis & Woodhams, unpublished) were deposited in the DDBJ/EMBL/GenBank databases. Some of the isolates are also available from the Culture Collection of Switzerland including a violacein-producing strain of Janthinobacterium lividum (http://www.ccos.ch/material/special_property_strains/violacein). Thus, accession numbers indicated in the metadata are limited to a minority of the isolates.
D4. Non-amphibian hosts not yet included
The current database contains information on bacteria associated with amphibian skin. The database could be expanded to include isolates from additional body locations, or microbes isolated from other organisms or environmental sources.
D5. Amphibian studies not included
In addition to the 18 studies included here (Table 2), there are several studies of antifungal bacteria from amphibian skin in progress. Additional published studies were not included if sequences were not made publically available. These include isolates from the skin of boreal toads, Anaxyrus boreas (Park et al. 2014).
D6. Differences in Bd strains
Because Bd is able to adapt to various culture conditions (Farrer et al. 2013, Voyles et al. 2014), we expect that if an amphibian host's symbiotic bacteria exerts similar selective pressures that different Bd strains may develop different levels of resistance to bacterial secondary metabolites. This has not yet been tested experimentally. Differences among Bd genomes have been noted along the path of chytridiomycosis emergence in North and Central America with evidence for selection acting on the Bd genome including chromosome copy number variation and loss of heterozygosity (James et al. 2009, Rosenblum et al. 2011, Farrer et al. 2013, Phillips and Puschendorf 2013).
Class III. Data set status and accessibility
A. Latest update: September, 2014.
This is the first version of this data set.
B. Latest metadata update
This is the first version of the metadata.
C. Copyright or Proprietary Restrictions
This data set is freely available for non-commercial scientific use, given the appropriate scholarly citation.
D. Contact person
Douglas C. Woodhams formerly at the University of Colorado, and currently at the University of Massachusetts Boston, Boston, MA, USA, [email protected].
Class IV. Data structural descriptors
A. Data set files
There are two data files. The first is a FASTA file that contains 16S rRNA sequences from each isolate. The sample ID in the FASTA file matches the sample ID in the metadata file. In addition to sample ID, the metadata file contains information from each isolate including the host-species, life-stage, geographic region, and antifungal capacity of the isolate, as well as the taxonomic classification and confidence value for the classification, and a GenBank accession number, if available.; The metadata is in tab delimited text format.
A.1. 16S rRNA sequences file: Amphibian-skin_bacteria_16S_sequences.fna
A.1.a. File format and size
FASTA format (2.2 Mb), 1944 sequences with headers indicating the isolate name matching the SampleID in the metadata file.
A.2. Metadata: Amphibian-skin_bacteria_metadata.txt
A.2.a. File format and size
Tab delimited text file (576 Kb) with a header row and 1944 entries (cultured bacterial isolates).
A.2.b. Fields
SampleID: This is a unique identifier for the cultured isolate and matches the identifier in the FASTA file indicating the 16S rRNA sequence. The identifier indicates the host species, the Bd-inhibition result, and a sequential number.
Original_SampleID: This is a unique identifier for the cultured isolate used in the original study.
Bd-inhibition: This column indicates whether the isolate was capable of significantly inhibiting (inhibitory) or enhancing (enhancing) Bd growth, was not significant (ns), or in some cases the result is unknown (unknown).
Host-species: The host species name is indicated.
Life-stage: Life-history stage of the host is categorized into Adult, Subadult, Tadpole, or Egg. Adults are full grown whereas Subadults are reproductively immature but post-metamorphosis.
Region: Geographic region indicated in Table 1.
Study: Research project indicated in Table 2.
RDP2.3_taxomony: The taxonomic classification is given according to the RDP classifier program, version 2.3.
RDP_confidence: This field indicates the quality score for the assignment provided by the RDP classifier as a confidence value.
Uclust_taxonomy: The taxonomic classification is given according to uclust in Qiime 1.8.0 using the GreenGenes 13_8 reference taxonomy.
Uclust_confidence: The confidence value for the taxonomic assignment given by uclust.
GenBank_accession: This field indicates an accession number for some isolates.
Mariannaea_inhibition: This field indicates whether the isolate was capable of inhibiting Mariannaea elegansgrowth (positive or weak inhibition noted), or no inhibition observed (negative). (Studies 13 and 14 only.)
Rhizomucor_inhibition: This field indicates whether the isolate was capable of inhibiting Rhizomucor variabilis growth (positive or weak inhibition noted), or no inhibition observed (negative). (Studies 13 and 14 only.)
Class V. Supplemental descriptors
There are no supplemental descriptors.
Acknowledgments
Thanks to Timothy M. Chappell for help collecting Rana sphenocephala isolates. Thanks to Janet Foley, Jonah Piovia-Scott, Tara Roth, and Joy Worth for providing Rana cascadae isolate information. Thanks to Andy Loudon for Janthinobacterium isolates and Graham Goodman for assistance with Bd assays. Thanks to Valeria Ramírez for assistance in the lab.
Literature cited
Anderson, P. K., A. A. Cunningham, N. G. Patel, F. J. Morales, P. R. Epstein, and P. Daszak. 2004. Emerging infectious diseases of plants: pathogen pollution, climate change and agrotechnology drivers. Trends in Ecology and Evolution19:535–544.
Antwis, R. E., R. L. Haworth, D. J. P. Engelmoer, V. Ogilvy, A. L. Fidgett, and R. F. Preziosi. 2014. Ex situ diet influences the bacterial community associated with the skin of red-eyed tree frogs (Agalychnis callidryas). PLoS ONE 9(1): e85563. doi:10.1371/journal.pone.0085563
Becker, M. H., R. N. Harris, K. P. Minbiole, C. R. Schwantes, L. A. Rollins-Smith, L. K. Reinert, R. M. Brucker, R. J. Domangue, and B. Gratwicke. 2011. Towards a better understanding of the use of probiotics for preventing chytridiomycosis in Panamanian golden frogs. Ecohealth 8:501-506.
Bell, S. C. 2012. The role of cutaneous bacteria in resistance of Australian tropical rainforest frogs to the amphibian chytrid fungus Batrachochytrium dendrobatidis. Ph.D. thesis, James Cook University.
Bell, S. C., R. A. Alford, S. Garland, G. Padilla, and A. D. Thomas. 2013. Screening bacterial metabolites for inhibitory effects against Batrachochytrium dendrobatidis using a spectrophotometric assay. Diseases of Aquatic Organisms 103:77-85.
Bletz, M. C., A. H. Loudon, M. H. Becker, S. C. Bell, D. C. Woodhams, K. P. Minbiole, R. N. Harris. 2013. Mitigating amphibian chytridiomycosis with bioaugmentation: characteristics of effective probiotics and strategies for their selection and use. Ecology Letters 16:807-820.
Caporaso, J. G., J. Kuczynski, J. Stombaugh, K. Bittinger, F. D. Bushman, E. K. Costello, N. Fierer, A. G. Peña, J. K. Goodrich, J. I. Gordon, G. A. Huttley, S. T. Kelley, D. Knights, J. E. Koenig, R. E. Ley, C. A. Lozupone, D. McDonald, B. D. Muegge, M. Pirrung, J. Reeder, J. R. Sevinsky, P. J. Turnbaugh, W. A. Walters, J. Widmann, T. Yatsunenko, J. Zaneveld, and R. Knight. 2010. QIIME allows analysis of high-throughput community sequencing data. Nature Methods 7:335–336. doi:10.1038/nmeth.f.303
Cornelison, C.T., K. T. Gabriel, C. Barlament, and S. A. Crow. 2014. Inhibition of Pseudogymnoascus destructans growth from conidia and mycelial extension by bacterially produced volatile organic compounds. Mycopathologia 177(1-2):1-10. doi: 10.1007/s11046-013-9716-2.
Daskin, J. H. 2011, March. Chytridiomycosis and symbiosis: context-dependency in amphibian disease and conservation. Master’s thesis. James Cook University. Available from http://eprints.jcu.edu.au/29935/
Daskin, J. H., S. C. Bell, L. Schwarzkopf, and R. A. Alford. 2014. Cool temperatures reduce antifungal activity of symbiotic bacteria of threatened amphibians--implications for disease management and patterns of decline. PLoS One 9(6):e100378. doi: 10.1371/journal.pone.0100378.
Farrer, R. A., D. A. Henk, T. W. J. Garner, F. Balloux, D. C. Woodhams, and M. C. Fisher. 2013. Chromosomal copy number variation, selection and uneven rates of recombination reveal cryptic genome diversity linked to pathogenicity. PLoS Genetics 9(8):e1003703.
Fisher, M. C., D. A. Henk, C. J. Briggs, J. S. Brownstein, L. C. Madoff, S. L. McCraw, and S. J. Gurr. 2012. Emerging fungal threats to animal, plant and ecosystem health. Nature 484:186-194.
Flechas, S. V., C. Sarmiento, M. E. Cárdenas, E. M. Medina, S. Restrepo, and A. Amézquita. 2012. Surviving chytridiomycosis: differential anti-Batrachochytrium dendrobatidis activity in bacterial isolates from three lowland species of Atelopus. PLoS ONE 7(9):e44832. doi:10.1371/journal.pone.0044832
Harris, R. N., R. M. Brucker, J. B. Walke, M. H. Becker, C. R. Schwantes, D. C. Flaherty, B. A. Lam, D. C. Woodhams, C. J. Briggs, V. T. Vredenburg, and K. P. Minbiole. 2009. Skin microbes on frogs prevent morbidity and mortality caused by a lethal skin fungus. ISME Journal 3:818-824.
Harris, R. N., T. Y. James, A. Lauer, M. A. Simon, and A. Patel. 2006. Amphibian pathogen Batrachochytrium dendrobatidis is inhibited by the cutaneous bacteria of amphibian species. Ecohealth 3:53–56.
Holden, W.M. 2014. The antifungal arsenal in amphibian skin: innate immune defenses against Batrachochytrium dendrobatidis in southern leopard frogs. Dissertation submitted to the graduate school of Vanderbilt University.
James, T. Y., A. P. Litvintseva, R. Vilgalys, J. A. T. Morgan, J. W. Taylor, M. C. Fisher, L. Berger, C. Weldon, L. du Preez, and J. E. Longcore. 2009. Rapid global expansion of the fungal disease chytridiomycosis into declining and healthy amphibian populations. PLoS Pathogens 5(5): e1000458. doi:10.1371/journal.ppat.1000458
Kamoun, S., O. Furzer, J. D. Jones, H. S. Judelson, G. S. Ali, R. J. Dalio, S. G. Roy, L. Schena, A. Zambounis, F. Panabières, D. Cahill, M. Ruocco, A. Figueiredo, X. R. Chen, J. Hulvey, R. Stam, K. Lamour, M. Gijzen, B. M. Tyler, N. J. Grünwald, M. S. Mukhtar, D. F. Tomé, M. Tör, G. Van den Ackerveken, J. McDowell, F. Daayf, W. E. Fry, H. Lindqvist-Kreuze, H. J. Meijer, B. Petre, J. Ristaino, K. Yoshida, P. R. Birch, F. Govers. 2014. The Top 10 oomycete pathogens in molecular plant pathology. Molecular Plant Pathology doi: 10.1111/mpp.12190.
Krugner-Higby, L., D. Haak, P. T. J. Johnson, J. D. Shields, W. M. Jones, K. S. Reece, T. Meinke, A. Gendron, and J. A. Rusak. 2010. Ulcerative disease outbreak in crayfish Orconectes propinquus linked to Saprolegnia australis in Big Muskellunge Lake, Wisconsin. Diseases of Aquatic Organisms 91:57–66.
Kueneman, J. G., L. W. Parfrey, D. C. Woodhams, H. M. Archer, R. Knight, and V. J. McKenzie. 2014. The amphibian skin microbiome across species, space and life history stages. Molecular Ecology 23:1238-1250. doi: 10.1111/mec.12510.
Küng, D., L. Bigler, L. R. Davis, B. Gratwicke, E. Griffith, and D. C. Woodhams. 2013. Stability of microbiota on a Panamanian frog: insights for probiotic therapy against disease. PLoS ONE 9(1): e87101. doi:10.1371/journal.pone.0087101.
Lam, B. A., J. B. Walke, V. T. Vredenburg, and R. N. Harris. 2010. Proportion of individuals with anti-Bactrachochytrium dendrobatidis skin bacteria is associated with population persistence in the frog Rana muscosa. Biological Conservation 143:529-531.
Lauer, A., M. A. Simon, J. L. Banning, E. Andre, K. Duncan, and R. N. Harris. 2007. Common cutaneous bacteria from the eastern red-backed salamander can inhibit pathogenic fungi. Copeia 3:630-640.
Lauer, A., M. A. Simon, J. L. Banning, B. A. Lam, and R. N. Harris. 2008. Diversity of cutaneous bacteria with antifungal activity isolated from female four-toed salamanders. ISME Journal 2:145-157.
Liu, Y., I. de Bruijn, A. L. Jack, K. Drynan, A. H. van den Berg, E. Thoen, V. Sandoval-Sierra, I. Skaar, P. van West, J. Diéguez-Uribeondo, M. van der Voort, R. Mendes, M. Mazzola, and J. M. Raaijmakers. 2014. Deciphering microbial landscapes of fish eggs to mitigate emerging diseases. ISME Journal doi:10.1038/ismej.2014.44.
McKenzie, V. J., R. M. Bowers, N. Fierer, R. Knight, and C. L. Lauber. 2012. Co-habiting amphibian species harbor unique skin bacterial communities in wild populations. ISME Journal 6:588-596.
Park, S. T., A. M. Collingwood, S. St-Hilaire, and P. P. Sheridan. 2014. Inhibition of Batrachochytrium dendrobatidis caused by bacteria isolated from the skin of boreal toads, Anaxyrus (Bufo) boreas boreas, from Grand Teton National Park, Wyoming, USA. Microbiology Insights 7:1-8. doi:10.4137/MBI.S13639
Perpiñán, D., J. G. Trupkiewicz, A. L., D. M. Geiser, S. Armstrong, M. M. Garner, and D. L. Armstrong. 2010. Dermatitis in captive Wyoming toads (Bufo baxteri) associated with Fusarium spp. Journal of Wildlife Diseases 46:1185-1195.
Phillips, A. J., V. L. Anderson, E. J. Robertson, C. J. Secombes, and P. van West. 2008. New insights into animal pathogenic oomycetes. Trends in Microbiology 16:13–19.
Phillips, B. L., and R. Puschendorf. 2013. Do pathogens become more virulent as they spread? Evidence from the amphibian declines in Central America. Proceedings Biological Sciences 280: 20131290. DOI:10.1098/rspb.2013.1290
Rosenblum, E. B., T. Y. James, K. R. Zamudio, T. J. Poorten, D. Ilut, D. Rodriguez, J. M. Eastman, K. Richards-Hrdlicka, S. Joneson, T. S. Jenkinson, J. E. Longcore, G. Parra Olea, L. F. Toledo, M. L. Arellano, E. M. Medina, S. Restrepo, S. V. Flechas, L. Berger, C. J. Briggs, and J. E. Stajich. 2013. Complex history of the amphibian-killing chytrid fungus revealed with genome resequencing data. Proceedings of the National Academy of Sciences U S A. 110(23):9385-9390.
Roth, T., J. Foley, J. Worth, J. Piovia-Scott, K. Pope, and S. Lawler. 2013. Bacterial flora on cascades frogs in the Klamath mountains of California. Comparative Immunology, Microbiology & Infectious Diseases 36:591–598.
Sarmiento-Ramirez, J. M., E. Abella, M. P. Martin, M. T. Telleria, L. F. Lopez-Jurado, A. Marco, and J. Diéguez-Uribeondo. 2010. Fusarium solani is responsible for mass mortalities in nests of loggerhead sea turtle, Caretta caretta, in Boavista, Cape Verde. FEMS Microbiology Letters 312:192–200.
Shaw, S. D., L. Berger, S. Bell, S. Dodd, T.Y. James, L. F. Skerratt, P. J. Bishop, and R. Speare. 2014. Baseline cutaneous bacteria of free-living New Zealand native frogs (Leiopelma archeyi and Leiopelma hochstetteri) and implications for their role in defense against the amphibian chytrid (Batrachochytrium dendrobatidis). Journal of Wildlife Diseases DOI:10.7589/2013-07-186
Skerratt, L.F., L. Berger, R. Speare, S. Cashins, K. R. McDonald, A. D. Phillott, H. B. Hines, and N. Kenyon. 2007. Spread of chytridiomycosis has caused the rapid global decline and extinction of frogs. Ecohealth 4:125-134.
Sutherland, W. J., R. Aveling, T. M. Brooks, M. Clout, L. V. Dicks, L. Fellman, E. Fleishman, D. W. Gibbons, B. Keim, F. Lickorish, K. A. Monk, D. Mortimer, L. S. Peck, J. Pretty, J. Rockström, J. P. Rodríguez, R. K. Smith, M. D. Spalding, F. H. Tonneijck, and A. R. Watkinson. 2014. A horizon scan of global conservation issues for 2014. Trends in Ecology and Evolution 29:15-22.
Van den Berg, A. H., D. McLaggan, J. Diéguez-Uribeondo, and P. Van West. 2013. The impact of the water moulds Saprolegnia diclina and Saprolegnia parasitica on natural ecosystems and the aquaculture industry. Fungal Biology 27:33–42.
Voyles, J., H. Johnson, C. Briggs, S. D. Cashins, R. A. Alford, L. Berger, L. Skerrat, R. Speare, and E. B. Rosenblum. 2014. Experimental evolution alters the rate and temporal pattern of population growth in Batrachochytrium dendrobatidis, a lethal fungal pathogen of amphibians. Journal of Ecology and Evolution DOI: 10.1002/ece3.1199
Walke, J. B., R. N. Harris, L. K. Reinert, L. A. Rollins-Smith, and D. C. Woodhams. 2011. Social immunity in amphibians: Evidence for vertical transmission of innate defenses. Biotropica 43:396-400.
Wang, Q, G. M. Garrity, J. M. Tiedje, and J. R. Cole. 2007. Naive bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Applied and Environmental Microbiology 73:5261-5267.
Woodhams, D. C., V. T. Vredenburg, M.-A. Simon, D. Billheimer, B. Shakhtour, Y. Shyr, C. J. Briggs, L. A. Rollins-Smith, and R. N. Harris. 2007. Symbiotic bacteria contribute to innate immune defenses of the threatened mountain yellow-legged frog, Rana muscosa. Biological Conservation 138:390-398.
Woodhams, D. C., J. Bosch, C. J. Briggs, S. Cashins, L. R. Davis, A. Lauer, E. Muths, R. Puschendorf, B. R. Schmidt, B. Sheafor, and J. Voyles. 2011. Mitigating amphibian disease: strategies to maintain wild populations and control chytridiomycosis. Frontiers in Zoology 8(1):8. doi: 10.1186/1742-9994-8-8.
Woodhams, D. C., H. Brandt, S. Baumgartner, J. Kielgast, E. Küpfer, U. Tobler, L. R. Davis, B. R. Schmidt, C. Bel, S. Hodel, R. Knight, and V. McKenzie. 2014. Interacting symbionts and immunity in the amphibian skin mucosome predict disease risk and probiotic effectiveness. PLoS One 9(4):e96375. doi: 10.1371/journal.pone.0096375.