Ecological Archives E096-020-A1
Carol M. Frost, Raphael K. Didham, Tatyana A. Rand, Guadalupe Peralta, and Jason M. Tylianakis. 2015. Community-level net spillover of natural enemies from managed to natural forest. Ecology 96:193–202. http://dx.doi.org/10.1890/14-0696.1
Appendix A. Supplementary methods: field site locations and description, Lepidoptera rearing, analyses of herbivore reduction efficacy and broken flight trap bias.
Supplementary Methods
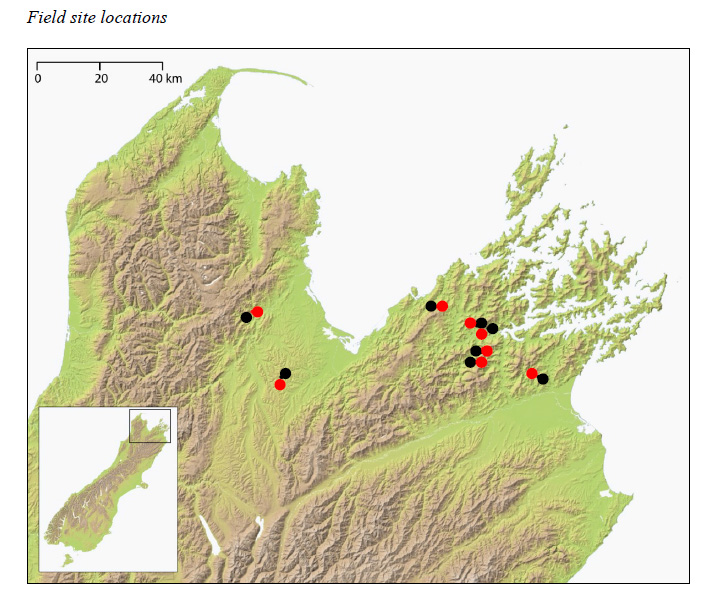
Fig. A1. Locations of field sites in the Nelson/Marlborough region of South Island, New Zealand. Black points represent control sites, and red points represent herbivore reduction treatment sites.
Field site vegetation description
Plantation forests (usually harvested at 28 years) were at least 17 years old, with mature pine trees and closed canopies, and care was taken to ensure that plantation age was as similar as possible at the two sites within each block. Plantation forest understorey vegetation ranged from dense to almost non-existent and included a range of native ferns, shrubs, and juvenile trees, with the most common species being Blechnum discolor, Pteridium esculentum, several species of Coprosma, Melicytus ramiflorus, Aristotelia serrata, Carpodetus serratus, Kunzea ericoides, and Leptospermum scoparium var. scoparium. Non-natives such as Ulex europaeus, Rubus fruticosus, and Leycesteria formosa were abundant in many sites, and where they were found, they were often the dominant understorey species. The native forest was dominated by the Nothofagaceae: Fuscospora fusca, F. solandri, F. truncata, and Lophozonia menziesii, as well as Weinmannia racemosa, with other broadleaf species and the occasional podocarp, such as Dacrydium cupressinum, and Dacrycarpus dacridioides making up the canopy. There were also patches in which Cyathea and Dicksonia tree ferns were the dominant canopy species. The understory vegetation in the native forest included the same native understorey species as in the plantation, as well as many other ferns and broadleaved shrubs. Occasionally the nonnative species indicated above were present, although these were never abundant.
Lepidopteran larva rearing methods
Immediately following collection, larvae were housed individually in small plastic cups, and fed an artificial diet for lepidopterans, specifically designed for beet army worm (Bio-Serv Entomology Custom Research Diets and Environmental Enrichment Products, New Jersey, USA), combined with fresh foliage of the plant species from which each larva was collected. Larvae were reared until they pupated and emerged as adult moths or until adult parasitoids emerged. The parasitoids were identified to species level (using Huddleston 1986, Austin 1992, Walker 1996, Berry 1997, van Achterberg 2004), or morphospecies (hereafter "species").
Analysis of the efficacy of the herbivore reduction treatment
We compared lepidopteran larva numbers before herbivore reduction (November collection) and after herbivore reduction (late January collection), on plantation and native sides of the edge at control and herbivore reduction sites, using a (GLMM) with Poisson errors. We included treatment (herbivore reduction versus control), forest type, collection (immediately before vs. immediately after herbivore reduction), and their interactions as fixed effects, as well as forest type, within site, within block as random factors. The best model was selected by running the full model as well as all possible simpler models, and selecting as the final model the one with the lowest Akaike Information Criterion (AIC) value (Burnham and Anderson 2002). We found that larval numbers did not differ significantly between control and herbivore reduction sites before herbivore reduction, but after herbivore reduction there was a significant reduction in larva numbers in the plantation but not in the adjacent native forest at herbivore reduction sites (interaction effect Z = -2.76, p = 0.006, Table A1). This demonstrates that our herbivore reduction treatment was effective.
Table A1. Coefficients of the best-fitting GLMM with Poisson errors (and log link function) in which caterpillar number was predicted, in the full model, by herbivore reduction treatment (herbivore reduction vs. control), forest type (plantation vs. native), collection (immediately before herbivore reduction vs. immediately after herbivore reduction), and their interactions, with forest type nested within site, nested within block as random effects. Significant P values (α ≤ 0.05) are indicated in bold.
Fixed effects |
Estimate |
Std. Error |
z value |
Pr(>|z|) |
Intercept (Before, Native, Control) |
3.1824 |
0.2830 |
11.24 |
<0.0001 |
Treatment (Herbivore reduction) |
0.4014 |
0.3958 |
1.01 |
0.3104 |
Forest (Plantation) |
-0.6504 |
0.3874 |
-1.68 |
0.0931 |
Collection (After) |
0.3467 |
0.3764 |
0.92 |
0.3570 |
Treatment*Forest (Herbivore reduction, Plantation) |
0.7411 |
0.5378 |
1.38 |
0.1683 |
Treatment*Collection (Herbivore reduction, After) |
-0.1999 |
0.5289 |
-0.38 |
0.7055 |
Forest*Collection (Plantation, After) |
0.3507 |
0.5389 |
0.65 |
0.5152 |
Treatment*Forest*Collection (Herbivore reduction, Plantation, After) |
-2.1043 |
0.7622 |
-2.76 |
0.0058 |
Fig. A2. Bi-directional malaise-style insect flight intercept trap. Insects flying from plantation-to-native forest vs. native-to-plantation forest were collected in separate jars. Photo credit: C. M. Frost.
Analysis of broken flight trap bias
To determine whether trap losses occurred in a biased way with respect to our treatments of interest, we used a generalized linear mixed model (GLMM) with a binomial error distribution to model occurrences of missing samples. We included as fixed effects herbivore reduction treatment, forest type, collection date, trap height, and all interactions with herbivore reduction treatment, and included forest type (native or plantation), nested within paired sites, nested within spatial blocks as random factors. Model selection was as above. We found that in the best-fitting model only collection date contributed significantly to the model, and contrasts between levels of collection showed no significant differences (Table A2).
Table A2. Coefficients from the best-fitting GLMM with binomial error (logit-linked) to determine whether incidence of broken traps was related to herbivore reduction treatment, forest type, collection, trap height, or any interactions with herbivore reduction treatment. Forest type within site within block were included as random factors. Collection was the only predictor retained in the final model. Collection 6 is used as the intercept condition in order to display contrasts for collection dates with the most widely-varying estimates. Significant P values (α ≤ 0.05) are indicated in bold. Only the intercept was significant, indicating that significantly more than zero traps were broken, however the number of broken traps did not differ significantly across collections or any of the treatments removed from the model. This indicates that trap damage was unlikely to have biased any of our hypothesis tests.
Fixed effects |
Estimate |
Std. Error |
z value |
Pr(>|z|) |
Intercept (collection 6) |
-3.1529 |
0.4718 |
-6.68 |
<0.0001 |
Collection 2 |
-0.3192 |
0.5826 |
-0.55 |
0.5838 |
Collection 3 |
-0.1493 |
0.5596 |
-0.27 |
0.7896 |
Collection 4 |
-17.8588 |
2640 |
-0.01 |
0.9946 |
Collection 5 |
-0.3192 |
0.5826 |
-0.55 |
0.5838 |
Collection 7 |
-17.8852 |
2680 |
-0.01 |
0.9947 |
Literature cited
Austin, A. D., and P. C. Dangerfield. 1992. Synopsis of Australasian Microgastrinae (Hymenoptera: Braconidae), with a key to genera and description of new taxa. Invertebrate Taxonomy 6:1–76.
Berry, J. A. 1997. Meteorus pulchricornis (Wesmael) (Hymenoptera: Braconidae: Euphorinae), a new record for New Zealand. New Zealand Entomologist 20:45–48.
Burnham K. P., and D. R. Anderson. 2002. Model selection and multimodel inference: a practical information-theoretic approach. Second Edition. Springer, New York, New York, USA.
Huddleston, T. 1986. The braconid genus Meteorus in New Zealand (Insecta: Hymenoptera). Journal of Natural History 20:255–265.
van Achterberg, C., L. Berndt, E. Brockerhoff, and J. Berry. 2004. A new species of genus Aleiodes Wesmael from New Zealand (Hymenoptera: Braconidae: Rogadinae). Zoologische Mededelingen 78:301–311.
Walker, A. K. 1996. A new species of Choeras (Braconidae: Microgastrinae) widespread in New Zealand. New Zealand Entomologist 19:43–48.