Ecological Archives E095-064-D1
Tyler R. Kartzinel, Jacob R. Goheen, Grace K. Charles, Elyse DeFranco, Janet E. Maclean, Tobias O. Otieno, Todd M. Palmer, and Robert M. Pringle. 2014. Plant and small-mammal responses to large-herbivore exclusion in an African savanna: five years of the UHURU experiment. Ecology 95:787. http://dx.doi.org/10.1890/13-1023.1
Introduction
Large mammalian herbivores (“LMH”, ≥ 5 kg) directly affect plant community structure, diversity, and function (Huntly 1991, Milchunas and Lauenroth 1993) and indirectly affect the abundance and diversity of other organisms (Keesing 1998, Pringle et al. 2007, Martin et al. 2010). Research on the ecological impacts of LMH is valuable for multiple disciplines of basic and applied research. These include understanding contemporary ecosystem dynamics (van Langevelde et al. 2003); evaluating the direct and indirect effects of consumers on plant assemblages, productivity, and ecosystem functioning (Bagchi and Ritchie 2011); testing hypotheses about the legacies of Pleistocene megafauna (Guimarães et al. 2008); predicting the impacts of declines and extirpation of LMH (Ogutu and Owen-Smith 2003), or of LMH hyper-abundance in some systems (e.g., Beguin et al. 2011); and making decisions about whether and how to manage LMH populations (Walker et al. 1987, Weisberg et al. 2002).
Due to difficulties inherent in the experimental manipulation of large animals, there are few data regarding two questions with major implications for all of the issues listed above. First, to what extent are different LMH size classes functionally redundant or distinct in terms of their effects on population, community, and ecosystem-level attributes? Existing studies addressing this question are mostly observational (but see: Young et al. 2005, Bakker et al. 2006, Staver et al. 2009), leading to difficulties in identifying causal mechanisms. Second, how do the direction and magnitude of LMH impacts vary across environmental gradients? Empirical tests of these questions often use meta-analytical approaches (Chase et al. 2000, Hillebrand et al. 2007). While these approaches are informative, they can also confound multiple aspects of environmental variation, divergent experimental methodologies and local species pools, and other attributes that inevitably differ between studies and locations. Experiments that simultaneously impose identical manipulations of LMH assemblages across environmental gradients within ecosystems, among sites that are similar in aspects other than the gradient of interest, are a needed bridge between small-scale mechanistic studies and broad meta-analytic syntheses (Gruner 2008). There have been few such studies to date (but see: Osem et al. 2004, Frank 2005, Anderson et al. 2007).
We present data from the first five years of an experiment that was designed to help resolve these two questions, which may also be useful in evaluating a range of other basic issues in ecology—for example, patterns of spatial variation and covariation among species within plant assemblages, relationships between understory and overstory savanna plants, and correlates of small-mammal density and diversity, to name but a few. The UHURU experiment (“UHURU”, for Ungulate Herbivory Under Rainfall Uncertainty) was established in 2008 to selectively exclude nested subsets of LMH within an assemblage consisting of at least 14 co-occurring species. Three features distinguish UHURU from prior experiments: (a) selective exclusion of successively smaller subsets of the LMH guild; (b) replication across an important ecological gradient (precipitation), but without substantial confounding variation in edaphic characteristics and species pools; and (c) plot sizes that are sufficiently large to evaluate indirect effects of LMH on smaller consumers such as insects and small mammals.
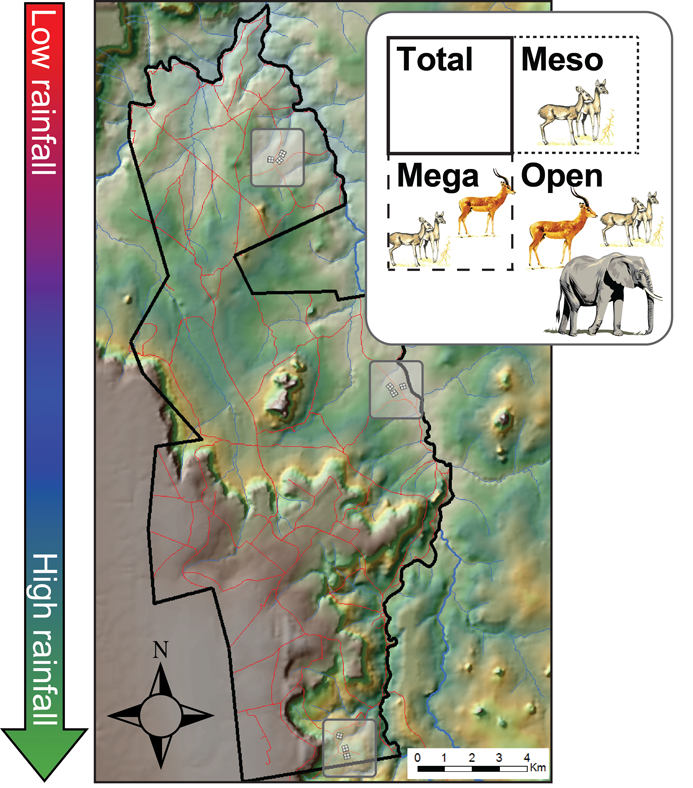
The experiment is located at the Mpala Research Centre and Conservancy in central Kenya (0º17’N, 37º52’ E, 1600 m above sea level; Fig. 1). The three exclusion treatments in UHURU are as follows: “Mega” (Fig. 2A) consists of electric wires strung 2 m from ground level, excluding only megaherbivores (elephants Loxodonta africana and giraffes Giraffa camelopardalis); “Meso” fences (Fig. 2B) exclude both megaherbivores and mesoherbivores (impala Aepyceros melampus, zebra Equus quagga and E. grevyi, waterbuck Kobus defassa, eland Taurotragus oryx, buffalo Syncerus caffer, oryx Oryx beisa, and gerenuk Litocranius walleri); and the “Total exclusion” treatment (Fig. 2C) excludes all herbivores ≥ 5 kg, a set that includes dik-dik (Madoqua cavendishi) and warthog (Phacochoerus africanus) in addition to the larger taxa listed above. Unfenced control plots (“Open”; Fig. 2D) are demarcated by short fence posts and are accessible to all native LMH species. In the center of each plot, we have established a permanent 60 × 60 m grid with stakes at 10-m intervals (49 total stakes; 0.36-ha total area), within which we conduct vegetation identification and measurements (herb abundance, woody vegetation size) as well as and small-mammal capture and release surveys.
Three randomized blocks, each containing one replicate of each fenced treatment and control, are located at each of three locations (which we refer to as “Sites”) across a natural rainfall gradient spanning 20 km (Fig. 1). Total annual rainfall increases from northern (“low-rainfall”; 2009–2012 range: 201–663 mm/y), through central (“intermediate-rainfall”; 235–808 mm/y), and southern sites (“high-rainfall”; 369–838 mm/y). Thus, depending on the year, the gradient encompasses a 27–84% increase in rainfall from the driest to the wettest site; by comparison, this is roughly equivalent to the increase from north to south across Kruger National Park (Gertenbach 1980), but at 1/18th of the distance. This pronounced variation in rainfall across a relatively short distance is the result of Mpala’s position in the rain shadow of Mt. Kenya, which lies approximately 60 km to the southeast.
We present 13 data sets containing results from floral and faunal response variables, as well as essential background data such as rainfall and large-herbivore dung counts, from 2008–2013. These raw data complement and extend the overview and preliminary analyses of Goheen et al. (2013). Specifically, these data sets encompass:
(1) The geographic coordinates of experimental plots.
(2) Rainfall at each site, monitored daily throughout the course of the experiment.
(3) Habitat characteristics at each of the 49 permanent stakes in each plot.
(4) Habitat characteristics in each 10 × 10 m cell of the 60 × 60 m grid in each plot.
(5) Biannual pin-frame surveys of understory plant diversity and abundance at 49 stakes/plot.
(6) Biannual surveys of understory composition within 49 small (0.25-m²) quadrats per plot.
(7) Biannual surveys of understory composition within 49 larger (1-m²) quadrats per plot.
(8) Annual size measurements of a subset of tagged and mapped trees within each plot.
(9) An initial comprehensive survey of the myrmecophyte Acacia drepanolobium in 2012.
(10) Annual censuses of overstory plant composition within the 60 × 60 m grid in each plot.
(11) Spatially-explicit overstory census data for each 10 × 10 m cell of the 60 × 60 m grid.
(12) Quarterly LMH dung surveys conducted in three parallel 60 × 5 m belt transects per plot.
(13) Small mammal mark-recapture data from Total and Open plots (6 trapping bouts/year).
The associated metadata describe monitoring protocols, along with refinements to these protocols that have been implemented as our understanding of the system has increased. These data profile the short-to-intermediate term ecological consequences of selectively excluding different nested subsets of a diverse LMH assemblage in a semi-arid African savanna ecosystem. We plan to periodically publish updated raw data for as long as we are able to maintain the experiment.
Fig. 1. Schematic of the UHURU experiment at the Mpala Research Centre and Conservancy in central Kenya (modified from Goheen et al. 2013). Black lines show the property boundary (the eastern side of which is the Ewaso Ng’iro river) and red lines show the network of dirt roads. Shaded grey boxes show the location of the three experimental sites, each of which contains three replicate blocks, each comprising four contiguous 1-ha plots. The inset depicts one block, which comprises one replicate of each exclusion treatment along with one control plot.
Fig. 2. Experimental treatment plots. (A) Megaherbivore fences consist of two parallel wires starting 2-m above ground level. (B) Mesoherbivore fences consist of 11 parallel wires starting ~0.3 m above ground level and continuing to 2.4-m above ground level. (C) Total-exclusion fences consist of 14 wires up to 2.4-m above ground level, with a 1-m high chain-link barrier at ground level. All fences are electrified using a solar charger and have a series of 1-m long electrified wires extending outwards to discourage animals from contacting the fence; Total- and Mesoherbivore-exclusion fences also have a series of short vertical wires to connect the parallel horizontal wires and add structural stability. (D) Open control plots are unfenced, with boundaries demarcated by short wooden posts at 10-m intervals. Photographs by E. DeFranco.
Metadata
Class I. Data set descriptors
A. Data set identity:
Title: Plant and small-mammal responses to large-herbivore exclusion in an African savanna: five years of the UHURU experiment
B. Data set identification code: NA
C. Data set description
Principal Investigators:
Robert M. Pringle, Department of Ecology and Evolutionary Biology, Princeton University, Princeton, New Jersey, United States of America.
Jacob R. Goheen, Department of Zoology and Physiology, University of Wyoming, Laramie, Wyoming, United States of America.
Questions regarding these data may be directed to: Robert Pringle ([email protected]).
Abstract: Assessing the direct and indirect consequences of nonrandom species removal within guilds of strongly interacting species, such as large mammalian herbivores, is an important goal in basic and applied ecology. The ecological impacts of such perturbations are often contingent on abiotic conditions, which have hindered efforts to generalize the results of field experiments. Thus, there is a need for experiments that selectively remove different species from ecologically important guilds and that are replicated across environmental gradients. In 2008, we constructed a series of size-selective large-herbivore exclosures across a natural rainfall gradient in semi-arid Kenyan savanna. This experiment (“UHURU”, for ungulate herbivory under rainfall uncertainty) aims to (a) characterize the effects of successively removing the largest size classes of herbivores from the system and (b) evaluate how the direction and magnitude of these effects are shaped by variation in precipitation regimes. UHURU consists of three electrically fenced herbivore-exclusion treatments and an unfenced control, applied to blocks of contiguous 1-ha plots. The three fenced treatments are: “Mega” (exclusion of elephants and giraffes only); “Meso” (exclusion of both megaherbivores and mesoherbivores, ca. 40 kg and larger); and “Total” (exclusion of all herbivores ≥ 5 kg). Each block of treatments is replicated three times at each of three sites along the 20-km rainfall gradient (increasing from 439 mm/yr in the north to 639 mm/yr in the south, with little background variation in soil attributes and species composition). We present data, spanning 2008 to 2013, from (a) biannual surveys of understory plants at 49 staked grid points within each of the 36 1-ha plots (1764 total stakes); (b) annual woody-plant censuses within the central 0.36 ha of each plot; (c) annual and semi-annual monitoring of individually marked woody plants; (d) small-mammal capture–mark–recapture sessions conducted every other month in total-exclusion and open plots; (e) daily rainfall monitoring throughout the course of the experiment; and (f) quarterly large-mammal dung surveys.
D. Key words: body size; climate; competition and facilitation; ecological field experiments; elephants; extinction; indirect species interactions; Kenya (East Africa); rodents; top-down control; ungulate herbivory.
Class II. Research origin descriptors
A. Overall project description
Originators: Jacob R. Goheen, Robert M. Pringle, Todd M. Palmer.
Period of study: 2008–2013. Continuing.
Objectives: To test predictions about the independent and interactive effects of large mammalian herbivore exclusion and rainfall variability on a broad range ecological response variables.
Abstract: As above.
Sources of funding: The UHURU experiment was built with seed funding from the Sherwood Family Foundation, grants from the National Sciences and Engineering Research Council of Canada (to JRG), and the Universities of Florida and British Columbia. Support for data collection has been contributed by the US National Science Foundation (DEB-0709880 and OISE-0852961 to RMP), the National Geographic Society, Stanford University’s Woods Institute for the Environment, the University of Wyoming, and the William F. Milton Fund of Harvard University.
B. Specific subproject description
Overview: The UHURU experiment excludes successively smaller-bodied nested subsets of large mammalian herbivores (≥ 5 kg, ranging in size from dik-dik Madoqua cavendishi to elephant Loxodonta africana). This design isolates the ecological impacts of different groups of herbivores and mimics a process of body size-biased large-herbivore extinction. Replicates spanning a 20-km rainfall gradient share similar edaphic characteristics and species pools. To test predictions about the independent and interactive effects of large mammalian herbivore exclusion and rainfall variability, a broad range of rainfall and habitat variables were recorded and a broad range of vegetation attributes and animal-population response variables were monitored.
Site locations and descriptions: The UHURU experiment is located at the Mpala Research Centre, Laikipia County, a semi-arid region of equatorial Kenya (0º17'N, 37º52' E, 1600 m above sea level). Mpala is in the rain shadow of Mt. Kenya, which imposes pronounced climatic variation across relatively small spatial scales: depending on the year, the increase in total annual rainfall from the northern (driest) site to the southern (wettest) site ranges from 27% to 84%.
C. Research methods
Experimental design: Beginning in 2008, a series of three herbivory treatments and a control were randomly assigned to contiguous 1-ha plots replicated three times at each of three sites along a rainfall gradient (36 total plots). Any field experiment entails a trade-off between plot size and replication. Plot sizes of 1 ha are larger than those in many prior large-herbivore-exclusion studies and enabled us to include nine replicates of each plot type, which was necessary both to sample multiple sites along the rainfall gradient and to achieve adequate statistical power. And although 1 ha is not large enough to detect all ecological effects of LMH (e.g., seed dispersal, numerical responses of smaller LMH to the exclusion of larger LMH), it is adequate to document strong and consistent effects in many individual-, population-, and community-level responses of plants and small mammals (Goheen et al. 2013), as well as behavioral (as opposed to numerical) responses of smaller LMH to the exclusion of larger LMH (e.g., Young et al. 2005). Total exclosures exclude all large mammalian herbivores (LMH) larger than ~5 kg and ~50-cm tall, but are accessible to hares and other small mammals; these exclosures use 2.4-m high fences consisting of 14 strands of wire, electrically charged by solar powered batteries, with a 1-m tall barrier consisting of 10-cm chain link. Mesoherbivore exclosures consist of 11 wires beginning 30-cm above the ground, allowing access to only the smallest LMH (dik-dik Madoqua cavendishi and warthog Phacochoerus africanus), and excluding larger species. Megaherbivore exclosures consist of two wires 2-m above ground level and exclude only megaherbivores (elephants Loxodonta africana and giraffes Giraffa camelopardalis). “Open” plots are unfenced and demarcated by a series of 1-m tall wooden posts at 10-m intervals; these plots allow access to all LMH species. On all fences, a series of 1-m long wires at 2-m height extend horizontally outward from plots to deter animals that approach the barriers. In January 2009, vertical connecting wires were added to total- and mesoherbivore exclusion fences to increase security and stability. Exclosure plots are regularly inspected and maintained by project personnel. Rapid repairs are made whenever damage to the fencing is discovered. For the eight most common LMH species, exclosure effectiveness ranged from 92% (for elephants) to 99% (for warthog and dik-dik; mean effectiveness for all LMH = 96%) during the first 3 years of the experiment (Goheen et al. 2013). Herdsmen for Mpala’s cattle-ranching operation are asked to keep cattle out of the plots. Within each plot, a 0.36-ha grid (60 × 60 m) marked by 49 rebar stakes at 10 m intervals provides a spatial template for much of the experimental monitoring.
Rainfall Monitoring: Rainfall has been continuously monitored from October 2008 onward at each of the three experimental sites (Goheen et al. 2013). Rainfall was originally measured using cylindrical drip gauges (All Weather Rain Gauge, Productive Alternatives, Fergus Falls, MN). A single automated tipping-bucket rain gauge (RainLogger, Rainwise Inc., Bar Harbor, ME) was installed in one of the total-exclusion plots at each site in June 2010, a second in July 2011, and a third in April 2012 (Goheen et al. 2013).
Habitat Surveys: Tree canopy cover, grass cover, and slope were assessed at each rebar stake and each 10 × 10 m square cell within the central 0.36-ha grid (grid cells are labeled corresponding to the rebar stake immediately southwest of the grid cell) in July–August 2011. Tree canopy cover was assessed directly above rebar stakes and grid squares. Tree species were recorded. We noted whether the trees in proximity to rebar were free-standing or part of a thicket. Levels of herbaceous cover, surface water features, and the prominence of sandy and stony substrates were also assessed.
Understory Monitoring: Grasses and forbs were surveyed biannually in February/March (dry season) and October (short rains). A 1-m² quadrat was placed immediately to the north of each of the 49 stakes demarcating the 0.36-ha center grid in each plot, and a 0.25-m² quadrat was placed within the larger quadrat. Species presence/absence was recorded within both quadrats. A 10-pinpoint frame was then positioned within the smaller quadrat, and the total number of vegetation pin hits was recorded for each species and/or the presence of bare soil. Individuals were identified to species (or to genus and morphospecies) using field guides and published species lists (Bogdan 1976, Blundell 1982, van Oudtshoorn 2009).
Tree Monitoring: Individual-based woody plant surveys focused on 10 tagged individuals per plot (or all individuals if there were less than 10 individuals per plot) of each of five common woody species, including the three dominant acacias (Acacia etbaica, A. mellifera, and A. brevispica), as well as Croton dichogamus (Euphorbiaceae), and Balanites aegyptiaca (Zygophyllaceae). Plants were tagged in January 2009, and tagged individuals were resurveyed in January each year. The following data were recorded: height (cm), basal diameter (mm) and/or circumference (cm) at 15 cm from ground level, and the number of stems at ground level.
In addition to the five common species, all A. drepanolobium individuals >1 m tall were tagged in southern plots in 2012. This species is monodominant on nearby black-cotton vertisol soils (Young et al. 1997), but is rare on sandier soils and restricted to the southern (high-rainfall) site in the UHURU experiment. In addition to the measurements described in the previous paragraph, we also recorded the number of flowers, floral buds, and fruits on each tree, as well as the associated ant-symbiont species (Young et al. 1997).
Each year, a census of all shrubs, trees, and tall succulents within the 0.36-ha plot centers was taken. Individuals were identified to species using field guides (Gillett and McDonald 1970, Dharani 2006) and assigned to one of five height classes (<1 m, 1–2 m, 2–3 m, 3–4 m, >4 m).
Mammal Monitoring: Surveys of LMH dung were conducted every three months to assess the efficacy of each experimental treatment in excluding the intended classes of LMH and to provide a relative index of LMH activity levels (Goheen et al. 2013). In each plot, three parallel 6 × 60 m belt transects (spaced 30 m apart within 0.36 ha plot centers) were walked by two observers, who counted all discrete dung piles and identified the species of origin according to the field guide of Stuart and Stuart (2000). Dung was crushed after identification to prevent its being recounted in subsequent surveys. Measurement of dung-decomposition rates at the three sites across the rainfall gradient suggest that assessments of relative LMH activity levels are unlikely to be biased by differential decay rates (Goheen et al. 2013).
Small mammals were live trapped at two-month intervals in total-exclusion and open plots using Sherman live-traps (Goheen et al. 2013). In each trapping session, and for four consecutive days, a single trap was set at each of the 49 grid stakes in the center of each plot, opened in the late afternoon, and checked and closed in the early morning. All species of small mammals were fit with two fingerling ear tags, with the exceptions of individuals in the genera Acomys, Crocidura, and Mus. These genera are too small or too fragile (Seifert et al. 2012) for ear tags; we instead marked individuals in these genera with black marker for subsequent identification within trapping sessions. Sample sizes and movement patterns by the four most commonly captured and marked small mammals (Aethomys hindei, Elephantulus rufescens, Gerbilliscus robustus, and Saccostomus mearnsi) –; represented by (a) the maximum distance moved by an individual within a four-day sampling period; (b) the probability of remaining on a sampling grid between successive periods from Program MARK (1-gamma”; White and Burnham 1999); and (c) the number of times (out of 16 sampling periods) an individual was captured on more than one plot – indicate that the 1-ha UHURU plot size is sufficiently large to observe effects of LMH exclusion on small mammals (Table 1). Initial misclassifications of Taterillus harringtoni as juvenile Gerbilliscus robustus were identified in May 2011 via DNA barcoding; we now distinguish between these two species based on hindfoot length (>34 mm for G. robustus), mass (>60 g for G. robustus), and tail (tufted for T. harringtoni, not tufted for G. robustus). We retroactively revised the initial misclassifications based on weights, such that individuals ≥60 g were reclassified as G. robustus and those ≤50 g as T. harringtoni, assuming they were adults. There are 2 – 4 Mus spp. and several Crocidura spp. that we cannot reliably distinguish in the field. Weight, sex, age, and reproductive condition were recorded for every captured individual.
Table 1. UHURU 1-ha plot sizes are large relative to the scale of movement by the four most commonly captured and marked small mammals within and between sampling periods. Data include the maximum distance moved within a four-day sampling bout, the probability of an individuals’ remaining within a sampling grid between successive sampling periods, and the total number of times that any individual has been captured in more than one plot between periods.
Species |
Maximum distance (sample size) |
Probability of |
Inter-plot |
Aethomys hindei |
22.6 (99) |
0.70 |
0 |
Elephantulus rufescens |
21.7 (23) |
0.51 |
1 |
Gerbilliscus robustus |
18.66 (57) |
0.57 |
2 |
Saccostomus mearnsi |
15.2 (74) |
0.78 |
0 |
Permit history: Kenya National Council for Science and Technology (Robert Pringle; NCST/5/002/R/656); University of Wyoming Institutional Animal Care and Use Committee Protocol Approval (Jacob Goheen; SKMBT_60112030515200; SKMBT_60112030515201; SKMBT_60112030515202; SKMBT_60112030515210).
Project personnel: The project employs several full-time Kenyan field assistants, who assist in the collection of data and maintenance of the experimental infrastructure. Ali Hassan, Samson Kurukura, Simon Lima, Jackson Lima, Antony Eshwah, and Mohamud Mohamed have worked in this capacity. In addition to the authors, Allison M. Louthan, Lucy Ngatia, Hillary S. Young, Stephen N. Kinyua, Brian F. Allan, and Claire Guinez have collected various data within UHURU.
Class III. Data set status and accessibility
A. Status
Latest update: May 2013.
Metadata status: The metadata are current and stored with the data.
B. Accessibility
Storage location and medium: Original data files exist on the authors’ personal computers, external hard drives, and Google Drive in MS Excel® format and .txt formats. Data format and the programs required to access and manipulate data will be kept current through the projected 20-year duration of this study. Original field data are stored safely with the authors at the Mpala Research Centre and with the senior author at Princeton University.
Contact person: Robert M. Pringle, e-mail: ([email protected]), Tel. 609-258-8273. Department of Ecology and Evolutionary Biology, Princeton University, Princeton, New Jersey, United States of America.
Proprietary restrictions: Given the ongoing nature of UHURU, we strongly recommend contacting the authors prior to using these data, as updated data may be available. Notification about when and how data are used will be appreciated, but is not mandated by the authors.
Costs: None.
Class IV. Data structural descriptors
There are 13 files that provide location, rainfall, habitat, vegetation, and animal data from the first five years of the UHURU experiment. There are several column headings that identify the scale and location of sampling, appearing in many of the 13 datasets that follow.
Label |
Attribute |
Definition |
Survey/Census |
Survey or Census number. |
Numeric. |
Year |
Year of sampling. |
2008–2013. |
Month |
Month of sampling. |
Month. |
Site |
Plot location. |
North (dry), Central (intermediate), South (wet). |
Block |
Replicate. |
Number 1–3. |
Treatment |
Excluded LMH guild. |
OPEN = open plots; MEGA = megaherbivore; MESO = meso- and megaherbivore; TOTAL = all LMH excluded; OUT = near to, but outside experimental plots (rarely used). |
Plot |
Unique plot identifiers. |
Comprises level, block, and treatment. |
Rebar |
Rebar name. |
Alphanumeric ID of rebar stakes (49 per plot) |
PLOT LOCATIONS
A. Data set file
B. Variable information
Column |
Attribute |
Definition |
3 |
UTM_X_COORD |
Longitude, UTM format |
4 |
UTM_Y_COORD |
Latitude, UTM format |
5 |
DD_X_COORD |
Longitude in decimal degrees |
6 |
DD_Y_COORD |
Latitude in decimal degrees |
C. Data anomalies
None known.
RAINFALL DATA
A. Data set file
B. Variable information
Column |
Attribute |
Definition |
1 |
Date |
Date in form of day-month-year. |
2–4 |
Daily rainfall (mm) |
Manual rain gauges; One in south, central, and northern locations. Reported until installment of automatic gauges. |
5–13 |
Daily rainfall (mm) |
Automatic rain gauges at each site in June 2010 (S2, C1, N1), July 2011 (S1, C2, N3), or April 2012 (S3, C3, N2). |
C. Data anomalies
The automatic gauge in Block Two of the South site ceased recording data on 19 July 2011 and was not replaced.
HABITAT DATA I – GRID-STAKE HABITAT SURVEYS
A. Data set file
B. Variable information
Column |
Attribute |
Definition |
Variable codes |
6 |
Tree_canopy |
Describes canopy cover directly above the rebar. |
Under = fully under canopy; by_under = under canopy, but near edge; by = at canopy edge; by_open = beside a tree canopy; open = not under or near a canopy. |
7 |
Thicket |
When a rebar is under or beside a tree, is the tree a part of a thicket? |
Y = yes; N = No. When a rebar is not under or beside a canopy, the value is N. |
8 |
Herbaceous_cover |
Describes density of herbaceous cover around the rebar (seven levels). |
Bare = no herbaceous cover; few-bare, few, med-few, med, med-high, and high are increasing levels of cover. |
9 |
Slope |
Slope (four levels).
|
Increasing levels of slope: flat, low, med, steep. |
10 |
Aspect |
The aspect of the slope (eight levels). |
Four cardinal directions (N = north, E = east, S = south, W = west), intermediate cardinal directions, or flat. |
11 |
Water_channel |
Proximity of rebar to surface water (11 classes). |
N = no water association by = beside a surface water runoff channel in = within a surface water runoff channel. For rebar by or in a surface water runoff channel, qualifiers include: Flow = water runs over the area without a distinct channel. Shallow, med, and deep = levels of channel depth. |
12 |
Stony |
Rebar in stony substrate. |
Y = yes; N = No. |
13 |
Sandy |
Rebar in sandy substrate. |
Y = yes; N = No. |
14–27 |
Species names |
Tree providing canopy cover. Note: hibiscus indicates patches, rather than individuals. |
Y = yes; N = No. Close = rebar in the open, but a canopy is nearby (1–2 m). |
C. Data anomalies
Rebar stakes that could not be located during surveys are coded as NA.
HABITAT DATA II – GRID-SQUARE SURVEYS
A. Data set file
B. Variable information
Column |
Attribute |
Definition |
Variable codes |
6 |
Tree_canopy |
Approximate % canopy cover for the square. |
Percent. |
7 |
Thicket |
Trees form a thicket. |
Y = yes; N = No. |
8 |
Bare |
Proportion of bare ground. |
Proportion. |
9 |
Herbaceous_height |
Relative height of herbaceous cover. |
Three increasing levels of height: low, med, high. |
10 |
Slope |
Slope (four levels).
|
Increasing levels of slope: flat, low, med, steep. |
11 |
Aspect |
The aspect of the slope (eight levels). |
Cardinal directions: N = north, E = east, S = south, W = west, and intermediates. |
12 |
Stony |
Rebar in stony substrate. |
Y = Yes; N = No. |
13 |
Sandy |
Rebar in sandy substrate. |
Y = Yes; N = No. |
14–17 |
Water features |
Indicates at least one instance of these water features: Water_flow, shallow_chan, med_chan, deep_chan, listed in order of increasing overland water flow. |
Y = Yes; N = No. |
18–34 |
Species names |
Indicate which tree species provide the canopy cover. Trees listed at genus level are considered morphospecies. |
Y = Yes; N = No. Close = rebar in the open with a canopy nearby (1–2 m). Euphorbia_spp, Hibiscus-spp, and Indigofera_biggive approximate % cover where abundant. |
C. Data anomalies
Grids missed during surveys are coded as NA.
VEGETATION DATA – PIN-FRAME SURVEYS
A. Data set file
B. Variable information
Column |
Attribute |
Definition |
Variable codes |
9 |
Bare_Ground |
Number of bare ground pinhits. |
Numeric 0–10. |
10 |
Binary_Bare |
Completely bare ground. |
0 = No; 1 = Yes. |
11–140 |
Species names |
Number of pinhits for each species. |
Numeric. |
141 |
sum_not_bare |
Count of vegetation contacting pins. |
Numeric 0–131. |
142 |
sr |
Species richness |
Numeric 0–9. |
C. Data anomalies
Taxonomic identities are considered provisional if labeled as morphospecies, as genus with “sp.”, or as “unknown.” Identification is pending ongoing taxonomic investigation and DNA barcoding. Twenty-five new taxa were identified since the original surveys, four initially undistinguished taxa were split in subsequent surveys (Aristida_lumped =Aristida congesta and Aristida kenyensis, survey 5; Indigofera_lumped = Indigofera_big and Indigofera_small, survey 5; Justicia_lumped = Justicia_white and Justicia_pink, survey 5; Melhania_lumped = Melhania_ovata and Melhania_velutina, survey 7), and one putative morphospecies was identified (Euphorbia_rare = Euphorbia crotonoides, survey 8). These taxa are listed as NA in surveys for which they were not recognized.
VEGETATION DATA – SMALL (0.25M2) QUADRATS
A. Data set file
B. Variable information
Column |
Attribute |
Definition |
Variable codes |
9 |
BARE_GROUND |
Bare small quadrat. |
S = Yes; 0 = No. |
10–161 |
Species names |
Presence of each species in the small quadrat. |
S = present. |
C. Data anomalies
Taxonomic identities are considered provisional if labeled as morphospecies, as genus with “sp.”, or as “unknown.” Identification is pending ongoing taxonomic investigation and DNA barcoding. Twenty-five new taxa were identified since the original surveys, four initially undistinguished taxa were split in subsequent surveys (Aristida_lumped =Aristida congesta and Aristida kenyensis, survey 5; Indigofera_lumped = Indigofera_big and Indigofera_small, survey 5; Justicia_lumped = Justicia_white and Justicia_pink, survey 5; Melhania_lumped = Melhania_ovata and Melhania_velutina, survey 7), and one putative morphospecies was identified (Euphorbia_rare = Euphorbia crotonoides, survey 8). These taxa are listed as NA in surveys for which they were not recognized.
VEGETATION DATA – LARGE (1-M2) QUADRATS
A. Data set file
B. Variable information
Column |
Attribute |
Definition |
Variable codes |
9 |
Bare_Ground |
Bare large quadrat. |
B = Yes; 0 = No. |
10–161 |
Species names |
The presence of each species in the large quadrat. |
B = present. |
C. Data anomalies
Taxonomic identities are considered provisional if labeled as morphospecies, as genus with “sp.”, or as “unknown.” Identification is pending ongoing taxonomic investigation and DNA barcoding. Twenty-five new taxa were identified since the original surveys, four initially undistinguished taxa were split in subsequent surveys (Aristida_lumped =Aristida congesta and Aristida kenyensis, survey 5; Indigofera_lumped = Indigofera_big and Indigofera_small, survey 5; Justicia_lumped = Justicia_white and Justicia_pink, survey 5; Melhania_lumped = Melhania_ovata and Melhania_velutina, survey 7), and one putative morphospecies was identified (Euphorbia_rare = Euphorbia crotonoides, survey 8). These taxa are listed as NA in surveys for which they were not recognized.
VEGETATION DATA – LONGITUDINAL TREE SURVEYS
A. Data set file
B. Variable information
Column |
Attribute |
Definition |
7 |
SPECIES |
The name of the tagged species. |
8 |
ORIGINAL_TAG |
Original permanent tag number applied in 2009. |
9 |
NEW_TAG |
Replacement tag numbers applied to 50 trees to ensure continuity. |
10 |
DEAD |
Whether the tree is dead (Y = Yes; N = No). |
11 |
HEIGHT |
Tree height (m). |
12 |
AXIS_1 |
Length of canopy extent (m) |
13 |
AXIS_2 |
Length of canopy perpendicular to AXIS_1 (m) |
14 |
CIRC |
Circumference of tree (cm). |
15 |
MEASUREMENT |
Indicates whether the circumference (CIRC) was directly measured (C) or calculated from direct measurements of diameter (D). |
16 |
STEMS |
Number of stems at ground level. |
C. Data anomalies
Re-measuring tree heights and circumferences can be slightly imprecise. To improve precision in circumference measurements, we began painting rings at the height of measurement in 2012. Within this data set, sources of imprecision could include: variability in how high up the stem the calipers/tape was placed, changes in measurement instrument (in 2009 and 2010 we measured diameter with digital calipers; from 2011 to 2013 we measured circumference with a tape measure), or the inadvertent measurement of the wrong basal stem on a tagged tree. Nonetheless, tree heights and diameters can in fact increase or decrease dramatically from year to year, due to damage by elephants, drought, etc. We scrutinized data and identified all trees with changes in height or circumference greater than two standard deviations (n = 180, or 11.7% of all trees for height; n = 134, or 8.8% of all trees for circumference) between any two consecutive surveys to identify and correct inadvertent miscalculations, transcription errors, or other verifiable mistakes.
VEGETATION DATA – ACACIA DREPANOLOBIUM SURVEYS
A. Data set file
B. Variable information
Column |
Attribute |
Definition |
7 |
ID |
The permanent ID tag number for the tree. |
8 |
HEIGHT |
Tree height (m) or dead. |
9 |
AXIS1 |
Canopy dimensions at longest axis (m). |
6 |
AXIS2 |
Canopy dimensions, perpendicular to AXIS1 (m). |
7 |
CIRC |
Basal circumference at 15 cm above ground (cm). |
8 |
Flowers |
Number of flowers. |
9 |
Buds |
Number of buds. |
10 |
Fruits |
Number of fruits. |
11 |
Ant |
The species of ant symbiont. CS = Crematogaster sjostedti CN = Crematogaster nigriceps E = Empty CM = Crematogaster mimosae TP = Tetraponera penzigi Multiple symbionts are separated by an underscore. |
C. Data anomalies
None known.
VEGETATION DATA – TREE CENSUS SUMMARY
A. Data set file
B. Variable information
Column |
Attribute |
Definition |
7 |
Species |
Tree species or morphospecies to genus level. |
8–12 |
Size class |
Trees / size class / subplot |
13 |
Sum |
Total trees / species / subplot |
C. Data anomalies
We do not present data from 2011, because a complete data set was never collected in that year. Four entries of tree species within plots were recorded without measurements. When tree species or subplots are not listed in a row, this carries the same implied meaning that the tree species is not present or that the subplot contains no trees.
VEGETATION DATA – TREE CENSUS DETAILED
A. Data set file
B. Variable information
Column |
Attribute |
Definition |
8 |
Species |
Tree species. Genus-level designations represent single morphospecies. |
9-13 |
Size classes |
The number of trees for the corresponding species in each size class |
14 |
Sum |
The total number of trees measured. |
C. Data anomalies
We do not present data from 2011, because a complete data set was never collected in that year. Four entries of tree species within plots were recorded without measurements. When tree species or subplots are not listed in a row, this carries the same implied meaning that the tree species is not present or that the subplot contains no trees. In 2009, there was an error in the recording of the original field data such that grid cells 2D and 2E within the South Block 3 Open plot could not be distinguished; as a result, trees from these two subplots are pooled under the rebar column entry of “2D&2E”.
ANIMAL DATA – DUNG SURVEYS
A. Data set file
B. Variable information
Column |
Attribute |
Definition |
6 |
Line |
Transect line number. |
7-33 |
Source of dung |
Species of origin and age (old vs. new dung assessed by color). |
C. Data anomalies
Dung of several taxa cannot be differentiated reliably in the field. These include hares (Lepus capensis and L. saxatilis), plains and Grevy’s zebra (Equus quagga and E. grevyi), African buffalo (Syncerus caffer) and domestic cows (Bos taurus). We made no effort to differentiate predator dung according to species; instead, we lumped them within the size classes large, medium, and small. A transcription error occurred when recording block number in the Central plots during the January 2011 survey. As a result, Block 2 and Block 3 are coded as NA to reflect the uncertainty.
ANIMAL DATA – SMALL MAMMAL SURVEYS
A. Data set file
B. Variable information
Column |
Attribute |
Definition |
4 |
DATE |
Transect line number. |
10 |
NIGHT |
Trap night (per site per survey). |
11 |
SPECIES |
Acke = Acomys kempi = Kemp’s spiny mouse Acpe = Acomys percivali = Percival’s spiny mouse Aehi = Aethomys hindei = Hinde’s rock rat Arna = Arvicanthus nairobae = Nairobi grass rat Arni = Arvicanthus niloticus = African grass rat Croc = Crocidura spp. = white-toothed shrews Dend = Dendromus spp. = climbing mice Elru = Elephantulus rufescens = rufous elephant shrew Geni = Gerbilliscus nigricaudus = black-tailed gerbil Gero = Gerbilliscus robustus = fringe-tailed gerbil Grdo = Grammomys dolichurus = woodland thicket rat Grmi = Graphiurus microtis = small-eared dormouse Mana = Mastomys natalensis = Natal multi-mammate rat Same = Saccostomus mearnsi = northern pouched mouse Taha = Taterillus harringtoni = Harrington’s tateril UArvi = Arvicanthus spp. = grass rats UMus = Mus spp. = pygmy mice X = trap closed but empty at checking Zehi = Zelatomys hildegardeae = Hildegarde’s broad-headed stink mouse ? = Unknown |
12 |
CAPTURE |
C = Capture; R = Released |
13 |
SEX |
F = Female; M = Male. |
14 |
CONDITION |
L = lactating N = none (no reproductive condition) P = pregnant PL = pregnant and lactating S = scrotal |
15 |
AGE |
A = Adult; S=Subadult; J = Juvenile. |
16 |
LEFT_HIND_FOOT |
Gerbils spp. and Mus spp. only |
17 |
LEFT_TAG |
Tag number at survey. |
18 |
ORIGINAL_TAG |
Original tags number. Particularly useful for cross-referencing with LEFT_TAG column when a tag was missing or replaced. |
19 |
ID |
Individual identifier. |
20 |
MARKS |
Number of ear tags. |
21 |
WEIGHT |
Weight (g). |
22 |
NOTES |
Indicate areas where individual identifications or measurement interpretations require caution. In particular, this column indicates if ID tags were lost or replaced, or if an individual escaped during evaluation. The condition of some individual captures could be consequential, such as individuals captured dead or with broken limbs. |
C. Data anomalies
Two individuals collected prior to May 2011, which could either be adult Taterillus harringtoni or juvenile Gerbilliscus robustus (50–60 g range), cannot be unambiguously assigned to species; these are listed as “?”. Blank entries indicate no data.
Class V. Supplemental descriptors
Quality Control: The authors have checked data accuracy. Data have undergone substantial checking and re-checking through the analysis and publication of a peer-reviewed paper (Goheen et al., 2013). Data were recorded in the field on paper, input into MS Excel®, and checked for outliers or omissions. Any known data anomalies are reported with the corresponding data set. Original data sheets are stored with the authors.
Publications:
Goheen, J. R., T. M. Palmer, G. K. Charles, K. M. Helgen, S. N. Kinyua, J. E. Maclean, B. L. Turner, H. S. Young, and R. M. Pringle. 2013. Piecewise disassembly of a large-herbivore community across a rainfall gradient: The UHURU experiment. PLoS One 8:e55192.
Goheen, J. R., S. D. Newsome, D. Boro, K. Fox-Dobbs, T. O. Otieno, T. M. Palmer, H. S. Young, and R. M. Pringle. Abundance-occupancy relationships and pathways to ecological generalism in a small-mammal community. In preparation.
Louthan, A. M., D. F. Doak, J. R. Goheen, T. M. Palmer, and R. M. Pringle. 2013. Climatic stress mediates the impacts of herbivory on plant population structure and components of individual fitness. Journal of Ecology 101:1074–1083.
Louthan, A. M., D. F. Doak, J. R. Goheen, T. M. Palmer, and R. M. Pringle. Integrating the stress-gradient hypothesis and plant apparency: Herbivory controls plant interactions in an arid savanna. In review.
Pringle, R. M., J. R. Goheen, T. M. Palmer, G. K. Charles, E. DeFranco, R. Hohbein A. T. Ford, B. Torto, and C. E. Tarnita. Herbivory as a complex interaction: direct, indirect, and net effects of browsers on Solanum campylacanthum in an African savanna. In review.
Young, H. S., D. J. McCauley, R. Dirzo, J. R. Goheen, B. Agwanda, A. W. Ferguson, S. N. Kinyua, M. M. McDonough, T. M. Palmer, R. M. Pringle, T. P. Young, and K. M. Helgen. Context dependent effects of land-use on small mammal communities in central Kenya. In review.
Young, H. S., McCauley, D. J., Helgen, K. M., Goheen, J. R., Otárola-Castillo, E., Palmer, T. M., Pringle, R. M., Young, T. P., Dirzo, R. 2013. Effects of mammalian herbivore declines on plant communities: observations and experiments in an African savanna. Journal of Ecology 101:1030–1041.
Literature cited
Blundell, M. 1982. The wild flowers of Kenya. Collins, London, UK.
Dharani, N. 2006. Field guide to Acacias of East Africa. Briza Publications, Pretoria, South Africa.
Gertenbach, W. P. D. 1980. Rainfall patterns in the Kruger National Park. Koedoe 23:35–44.
Gruner, D. S., Smith, J. E., Seabloom, E. W., Sandin, S. A., Ngai, J. T., Hillebrand, H., Harpole, W. S., Elser, J. J., Cleland, E. E., Bracken, M. E. S., Borer, E. T. and Bolker, B. M. 2008. A cross-system synthesis of consumer and nutrient resource control on producer biomass. Ecology Letters, 11:740–755.
Staver, A. C., W. J. Bond, W. D. Stock, S. J. van Rensburg, and M. S. Waldram. 2009. Browsing and fire interact to suppress tree density in African savanna. Ecological Applications 19:1909–1919.
White G. C., and K. P. Burnham. 1999. Program MARK: Survival estimation from populations of marked animals. Bird Study 46:120–138.
Young, T. P., T. M. Palmer, and M. E. Gadd. 2005. Competition and compensation among cattle, zebras, and elephants in a semi-arid savanna in Laikipia, Kenya. Biological Conservation 122:351–359.