Ecological Archives E093-059-D1
Asem A. Akhmetzhanova, Nadejda A. Soudzilovskaia, Vladimir G. Onipchenko, Will K. Cornwell, Vladimir A. Agafonov, Ivan A. Selivanov, and Johannes H. C. Cornelissen. 2012. A rediscovered treasure: mycorrhizal intensity database for 3000 vascular plants species across the former Soviet Union. Ecology 93:689.
The mycorrhizal symbiosis is important for carbon and nutrient cycling in most of the world's ecosystems (Read 1991). According to different estimations, 80–94% of the vascular plant species worldwide have mycorrhizal associations (van der Heijden and Sanders 2002, Brundrett 2009). Due to their ability to access both mobile and immobile forms of nitrogen and phosphorus, plant–fungus symbioses are important for the nutrient acquisition of plants (Smith S.E. 2009). Besides nutrient acquisition, mycorrhizal fungi facilitate plant water relations (Auge 2001), protect plants against pathogens (Newsham et al. 1995, Borowicz 2001), and reduce uptake of toxic metals (Gadd 1993, Meharg 2003).
The extent of mycorrhizal infection intensity in a plant can vary depending on the species, climatic and soil conditions, and host community (Brundrett 1991, Postma et al. 2007, Becklin and Galen 2009). Elucidating these relations is crucial for understanding and predicting biogeochemical cycling. Despite several intensive investigations and syntheses (e.g. Read and Haselwandter 1981, Muthukumar and Udaiyan 2000, Treseder and Cross 2006, Wang and Qiu 2006, Brundrett 2009), the type and level of infection by mycorrhizal fungi is unknown for the great majority of the world's vascular plants. Large data compilations providing data for the same species in multiple habitats are not available yet. Here we present a previously unpublished large dataset on mycorrhizal type and infection rates of multiple species in multiple sites throughout the territory of the former Soviet Union (Fig. 1).
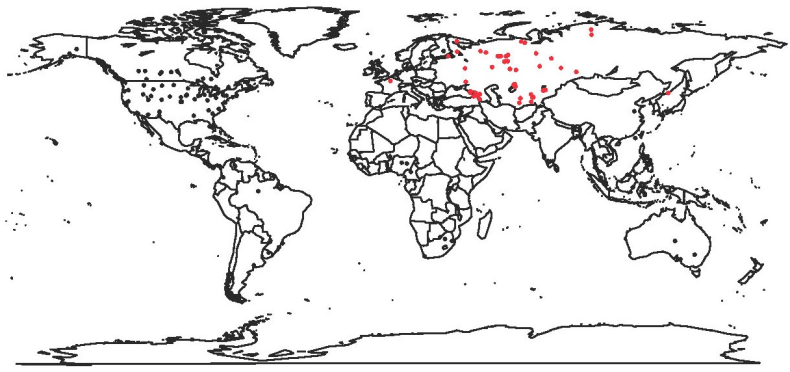
Fig 1. World distribution of AM mycorrhizal studies. Black points represent currently available data (Treseder and Cross 2006). Red points represent the data available in this database. Click image for larger version.
Our new database fills a huge data gap for central and northeast Asia while more than doubling such information currently in the global literature. Moreover, it presents the data in a format ready for widespread use and is accompanied by key environmental data. The availability of this database, when coupled to other plant trait, ecological, evolutionary, soil and climate data (e.g. Wang et al. 2010, Grunwald et al. 2011, Kattge et al. 2011), will help to generate and answer important and large-scale questions concerning biogeochemical cycling, climate change impact, interspecific versus intraspecific trait variation and co-evolution of plants and fungi.
In the period of 1950–1980 Russian professor Ivan Aleksandrovich Selivanov and his team of researchers and students (University of Perm, now Russia) collected a huge amount of hard data on natural mycorrhizal infection type and intensity. The dataset features 2970 plant species from 155 families in 154 sites representing the main plant communities across the former Soviet Union, mostly on the territory of modern-day Russia, Ukraine and Kazakhstan. This information (adding up to 7445 species by site records) was accompanied by extensive descriptions of plant community composition and site characteristics. For many species the intensity of mycorrhizal infection was quantified in multiple plant communities, thus providing a unique opportunity for a large-scale analysis on community-type dependence of mycorrhizal intensity for a large number of species. However, this information had been deposited only in an appendix of a large, non-digital thesis in Russian (Selivanov 1976). The methods and some summarized preliminary findings, largely qualitative, were published in a book (Selivanov 1981) and several journal publications in Russian. The latter, although distributed within in the former Soviet Union, have been inaccessible to the international research community, the more so because Prof. Selivanov himself died in 1998.
We extracted the raw data from the appendixes of the thesis of I.A. Selivanov, translated, modernized, and digitized it. Prof. Selivanov developed his own mycorrhizal type classification terminology, and his own method of estimation of mycorrhizal intensity, being comparable to the widely accepted method of McGonigle et. al (1990) but more precise and labor-intensive. We transformed the terminology used in the thesis into an internationally recognized one (see the Methods section for details). Furthermore we conducted our own investigation of mycorrhizal infection intensity on 66 plant species in the sub-Arctic (Abisko area, N Sweden) and the alpine zone of the Caucasus (SW-Russia) using both the method of McGonigle et al. (1990) and the method of Selivanov (see Methods section for details), and derived a simple equation for data conversion between the two methods.
A. Data set title: Database of mycorrhizal intensity for 2970 vascular plant species across multiple sites on the former Soviet Union territory
B. Data set identification code: myco_db.zip
C. Data set updates: The dataset is complete and no updates are planned.
D. Principal investigators: Same as authors.
E. Abstract
The symbiosis between vascular plants and mycorrhizal fungi is paramount for carbon and nutrient cycling in most of the world’s ecosystems. Most vascular plant species are associated with mycorrhizal fungal partners, and the association is essential for the carbon and nutrition economies of both partners. However, despite its clear importance, data on this symbiosis are lacking: for most vascular plant species, mycorrhizal type is unknown. Very rarely is there data on the levels of mycorrhizal infection intensity in multiple habitats.
We translated and digitized a huge data set on vascular plant mycorrhizal intensity throughout the former Soviet Union, previously available only as a hard copy appendix of the doctoral thesis of Ivan A. Selivanov published in Russian in 1976 and not accessible to the international research community. We updated the taxonomic plant nomenclature to the International Plant Name Index and adjusted mycorrhizal and ecological terminology according to the modern international literature.
The database contains 7445 records on mycorrhizal infection type and intensity of 2970 plant species from 155 families, in 154 sites, situated across the former Soviet Union (mostly on the territory of the current Russia, Ukraine, and Kazakhstan), comprising together extensive geological, topographic, and climatic gradients. The data set includes percentage infection for each species–site combination for arbuscular, ericoid, arbutoid, endo-mycorrhizal, dark septate, orchid- and ecto-mycorrhizal fungi. Each record has a detailed description of geography. For many records, soils and host plant community are described. Most of the sites are natural; 10 sites are situated in botanical gardens. For 1291 species the intensity of mycorrhizal infection is quantified in multiple plant communities (2–57). The remaining species are described at single sites.
Selivanov developed his own methods for quantifying mycorrhizal infection intensity. These methods are comparable, but not identical, to the methodology commonly used today. Based on our own sampling of 99 plant species collected in two distant sites (Caucasus [Russia] and Abisko [Sweden]), we provide a simple equation for data conversion between the two methods.
The availability of this database will help to provide answers to important questions concerning biogeochemical cycling, climate change impacts, and co-evolution of plants and fungi.
Key words: arbuscular mycorrhiza; carbon cycling; ecto-mycorrhiza; ericoid mycorrhiza; Kazakhstan; mycorrhizal infection; mycorrhizal type; nutrient cycling; root colonization; Russia; symbiosis; Ukraine
F. Key words: arbuscular mycorrhiza; carbon cycling; ecto-mycorrhiza; ericoid mycorrhiza; Kazakhstan; mycorrhizal infection; mycorrhizal type; nutrient cycling; root colonization; Russia; symbiosis; Ukraine
A. Overall project description: We translated, modernized and digitized the dataset of mycorrhizal intensity for plants of the territory of the former Soviet Union, earlier available only as a hard copy of a doctoral thesis of Prof. Selivanov being published in Russian.
B. Period of Study: 1957–2010 (1957–1975 for main data collecting, 2007–2010 for smaller calibration dataset)
C. Geography: The territory of the former Soviet Union.
D. Research motivation
The mycorrhizal symbiosis is possessed by a great majority of the world vascular plant species, being a crucially important contributor to plant nutrition and ecosystem carbon and nutrient cycling. Our dataset covers most of central and northeastern Eurasia, for which virtually no data on vascular plant mycorrhizal infection intensity have been available till now. Our database will help to answer important and large-scale questions concerning biogeochemical cycling, climate change impact, interspecific versus intraspecific trait variation and co-evolution of plants and fungi.
E. Research methods
Methods for determination of mycorrhizal intensity in vascular plants (adapted from Selivanov (1981))
Sampling
The investigation was conducted during the period 1957–1975. Sampling was conducted on 154 sites, located in a wide range of geographical zones from tundra to desert, see Fig. 2. For a detailed description of the sites, see file sites.txt. The great majority of the sites are natural habitats. Ten sites are in botanical gardens. We opted to include these sites into the database, however, the data from them should be used with caution, because growth conditions of plants in botanical gardens deviate from natural conditions, which could in turn affect mycorrhizal colonization.
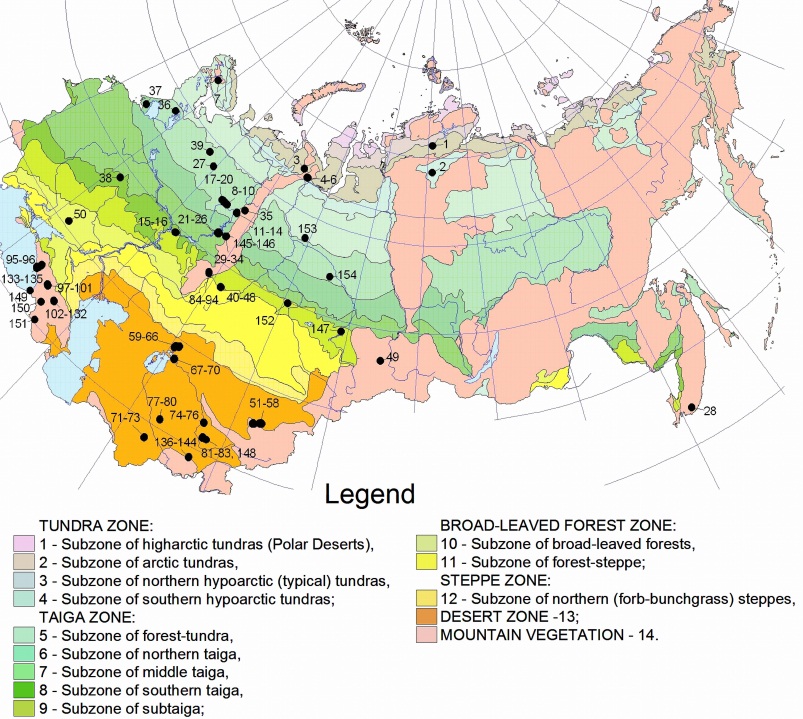
Fig. 2. Sampling site locations. Numbers on the map correspond to the site numbers in files myco_db.txt and sites.txt. Numbers behind zone names in the legend correspond to the climatic zone numbers in the file sites.txt. Click image for larger version.
Plant roots were collected during summer (June–August). For every plant species roots of 5–10 individual plants were dug out. For plant with fibrous roots the whole root system was taken. For plants with tap roots, only thin roots (< 2 mm in diameter) were taken. For mature trees, roots were followed from the trunk for 10–12 m, to be sure that the right individual was sampled, and thin roots of 2nd and 3rd order were taken at 5–20 cm depth. Roots were fixed in 4% formaldehyde solution (herbaceous plants) or in Carnoy's solution (trees) till lab analysis.
Analysis of mycorrhizal infection intensity
The per site, per species intensity of mycorrhizal infection was estimated in 50–500 individual root subsamples collected from at least 5 plants, and each subsample was graded into one of the classes, 0 to 5 according to infection intensity, the estimation of which depended on mycorrhiza type (see below).
The total per species rate of mycorrhizal infection MI (0 < MI < 100) was calculated individually for each site as:
MI = S / (N × K)
where S is the sum of grades for all considered root samples, N is the number of root samples, and K is the highest point of the scale (K = 5).
Estimation of mycorrhizal infection intensity in individual root samples for plants with arbuscular, orchid, dark septate (DS), and unidentified endo-mycorrhizas.
Fixed roots were macerated in 20% KOH for 2–3 months, then moistened and stained with aniline blue (0.1 g aniline blue and 50 g lactic acid per 100 ml water) for 1 hour. After removal from the stain solution the samples were put in pure lactic acid for one hour and subsequently kept in glycerol until microscopic analysis.
For each species 250–500 2-mm intersects along the root length of thin roots (diameter 300–600 µm) and 250–500 2-mm intersects of ultra-thin roots (< 300 µm) were analyzed microscopically (x120). On each intersect the amount of fungi was estimated and graded 0 to 5 as follows (see also Fig. 3):
0: No cortical cells contain mycorrhizal fungi
1: Mycorrhizal fungi are present in few cortical cells
2: About 25% of the cortical cells are occupied by fungi
3: About 50% of the cortical cells are occupied by fungi
4: About 75% of the cortical cells are occupied by fungi
5: Practically all of the cortical cells are occupied by fungi
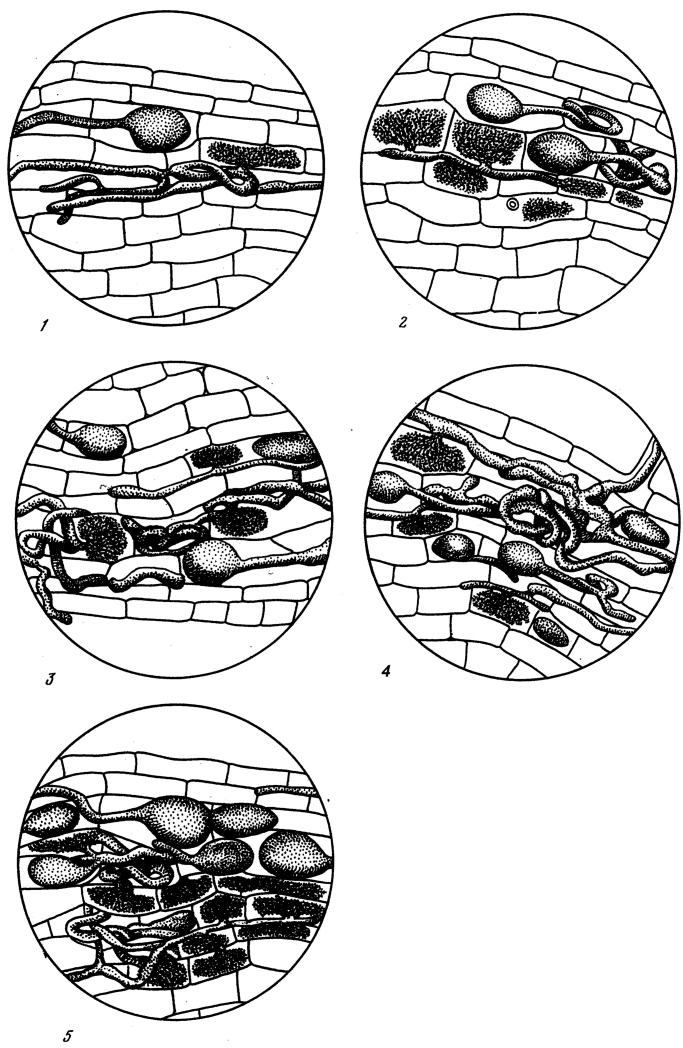
Fig. 3. Grading criteria (grades 1–5 from lowest to highest) for arbuscular, orchid, DS, and unidentified endo-mycorrhizas. Adapted from (Selivanov 1981).
Estimation of mycorrhizal infection intensity in individual root samples for plants with ericoid and arbutoid mycorrhizas.
Fixed roots were stained with aniline blue. After removal from the stain solution the samples were put in pure lactic acid for one hour and subsequently kept in glycerol till analysis.
From each species 200–500 root cross-sections from at least 5 plants were prepared and analyzed microscopically (x120). The amount of fungi visible in each cross-section was graded from 0 to 5 as follows (see also Fig. 4):
0: No hyphae are visible.
1: A few spaced hyphae are present on the root surface. A few individual cortical cells contain fungus.
2: There are separate infrequent hyphae on the root surface. About 50% of the root epidermis cells do not contain fungus. Cells of cortex inner layers do not contain fungus.
3: Root surface is covered by a loose network of hyphae. About 30% of the cortical cells do not contain fungus.
4: Root surface is covered by hyphal mantle. Fungus is abundant in cortical cells, but not all the cells contain fungi (2–3 cells are fungus-free).
5: Root surface is completely covered by dense hyphal mantle. Nearly all cortical cells contain fungus.
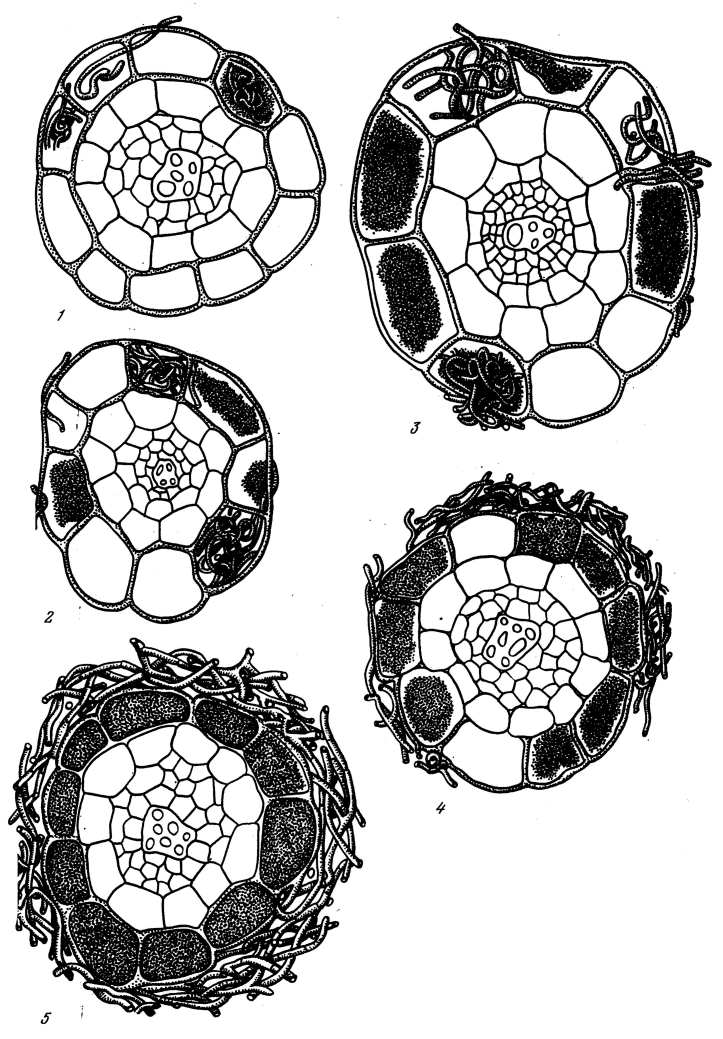
Fig. 4. Grading criteria (grades 1–5 from lowest to highest) for ericoid and arbutoid mycorrhizas. Adapted from (Selivanov 1981).
Estimation of mycorrhizal infection intensity in individual root samples for plants with ectomycorrhiza
The presence of ectomycorrhizas was detected by microscopic examination of cleared roots stained with safranin-lichtgrün (light green safranin), 1% alcoholic solution, for Hartig net observation. For detection of mycorrhizal infection intensity, the total number of mycorrhizal root tips per 100 mm of root was calculated. For each species 25–50 root samples were taken from 5–10 species. Each sample was assigned a grade from 0 to 5 based on the number of mycorrhizal root tips as follows:
0: No mycorrhizal root tips;
1: 0–10 mycorrhizal tips per 100 mm root;
2: 11–20 mycorrhizal tips per 100 mm root;
3: 21–30 mycorrhizal tips per 100 mm root;
4: 31–40 mycorrhizal tips per 100 mm root;
5: More than 40 mycorrhizal root tips per 100 mm of root.
Note that this method differs from the method widely used nowadays. In the modern literature often a percentage of colonized root tips is reported.
Methods for database creation
The data on per-site, per-species mycorrhizal infection type and intensity, as well as information about the site location, and description of the collection sites was found in an appendix to the Doctoral thesis of Selivanov (Selivanov 1976). This data was digitized and checked for consistency. Terminology used by Selivanov for distinct types of mycorrhizae was translated into an internationally recognized one according to (Smith and Read 2009) based on the detailed descriptions of terms given in the thesis.
Calibration between the methods of determination of arbuscular mycorrhizal infection intensity proposed by Selivanov (1981) and by McGonigle et al (1990)
The currently widely accepted method of McGonigle et al (1990) for determination of intensity of arbuscular mycorrhizal infection differs slightly from that of Selivanov (1981). McGonigle et al (1990) examine root intersects along the root length for the presence or absence of AM structures and then calculate the percentage of the intersects containing AM structures. In contrast, Selivanov (1981) takes into account the intensity of the fungal colonization in each such intersect. This method is somewhat similar to the "slide-length" method discussed by (Giovannetti and Mosse 1980), who also calculated the total infection value taking into account infection in individual root intersects. However the calculation details of the slide-length method and the method of Selivanov (Selivanov 1981) differ. In order to assess the compatibility of these two methods we performed our own sampling of 99 plant species in two distant natural sites: (1) the alpine belt of Caucasus, NW Russia, on Mt. Malaya Khatipara (4°26'N, 41°41'E) along an altitudinal gradient from forest to alpine zone, (1800–3500 m a.s.l), and (2) in the Abisko area, North Sweden (68°21'N, 18°49'E) in a variety of sites located at 350–1100 m a.s.l.
We collected roots of 5–10 plants for each species and processed them following the protocol described above for arbuscular mycorrhiza. Each species was collected on one site only. For each species we determined mycorrhizal intensity (MI) following Selivanov (1981) and the percentage root length colonized (RLC) following (McGonigle et al. 1990). For each plant species 250 intersections along the root length were examined under the microscope at x200 magnification.
To develop a way to convert MI to RLC, we did a logistic regression of RLC (response variable) on MI (predictor). Due to essentially binomial distribution of the data expressed as percentages, the logistic model provides the best possible match of the error structure of the data (Sokal and Rolf 1994); furthermore, the bounded nature (between 0 and 1) of logistic regression matches the nature of percentage data.
The regression showed a very tight fit between the two data sets, residual deviance was 8.5457 on 97 degrees of freedom compared to a total deviance of 65.7514 on 98 degrees of freedom. P < 10-6, Fig 5. The resulting equation for data conversion is:
RLC = 100 × exp(a + b × MI) / (1 + exp(a + b × MI))
Where b = 0.11741 and a = -2.788. Both RLC and MI are expressed in percent. Note that this equation should be applied for non-zero data, while values of MI = 0, should be manually converted to RLC = 0.
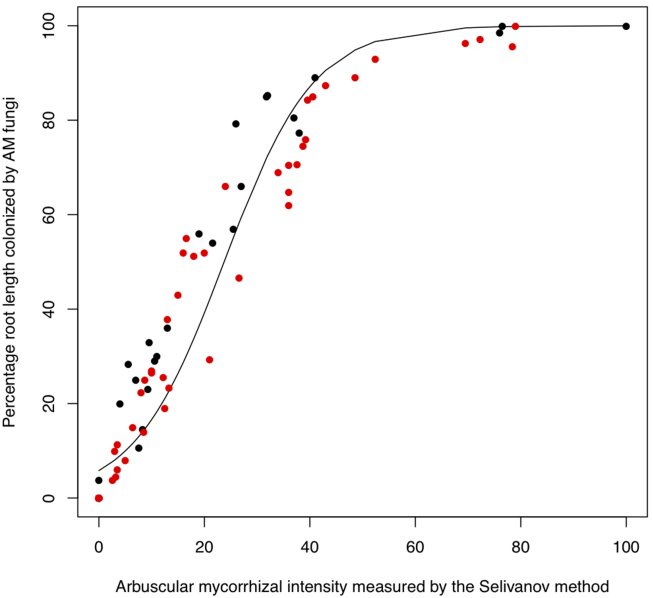
Fig. 5. Relationship between percentage root length colonized by arbuscular mycorrhizal fungi measured with the method of (McGonigle et al. 1990) and arbuscular mycorrhizal intensity measured by the method of Selivanov (1981) for 99 subarctic and alpine species. Red dots represent Caucasus data; black dots represent Abisko data
A. Status
Latest update: 28 July 2011
Metadata status: Metadata is complete.
Data verification: The data was checked for consistency. Species names were thoroughly checked and corrected according to The Plant List (2010). Genus and family level nomenclature follows the phylogeny of Angiosperm Phylogeny Group 3 (Stevens 2008).
B. Accessibility
Storage location and medium: The Ecological Society of America's Ecological Archives
C. Contact persons
Nadejda Soudzilovskaia
Systems Ecology
Institute of Ecological Science
Faculty of Earth and Life Sciences
VU University
De Boelelaan 1085
NL-1081 HV Amsterdam
The Netherlands
E-mail: [email protected]
Vladimir Onipchenko
Department of Geobotany
Faculty of Biology
Moscow State University
RU-119991 Moscow
Russia
E-mail: [email protected]
Hans Cornelissen
Systems Ecology
Institute of Ecological Science
Faculty of Earth and Life Sciences
VU University
De Boelelaan 1085
NL-1081 HV Amsterdam
The Netherlands
E-mail: [email protected]
D. Copyright restrictions: Any paper using the data should cite this paper.
Data on plant mycorrhizal intensity
A. Data Set File
Identity: Myco_db.csv
Size: 578000 bytes
Format and storage mode: ASCII text, comma separated
B. Header information
Number: Unique number of a record
Family: Vascular plant family name according to The Plant List (2010)
Genus: Vascular plant genus name according to The Plant List (2010)
Specific epithet: Vascular plant specific epithet according to The Plant List (2010)
Species: Binomial species name for vascular plant
Original term for mycorrhizal type given by Selivanov: Original term for mycorrhizal type given by Selivanov in his thesis. This field can have any of the following abbreviations:
ch.ect.: Eumycetic chalmophagic ectomycorrhiza, corresponds to Ectomycorrhiza in modern terminology
E.t.ect.arb.: Eumycetic tolypophagic ectomycorrhiza (arbutoid), corresponds to Arbutoid in modern terminology
E.t.ect.er.: Eumycetic tolypophagic ectomycorrhiza (ericoid), corresponds to Ericoid in modern terminology
E.t.end.: Eumycetic tolypophagic endomycorrhiza (orchid), corresponds to Orchid in modern terminology
Ecto: Ectomycorrhiza of unidentified type, corresponds to Ectomycorrhiza in modern terminology
Ecto arbut.: Ectomycorrhiza of arbutoid type, corresponds to Arbutoid in modern terminology
Ecto er.: Ectomycorrhiza of ericoid type, corresponds to Ericoid in modern terminology
Endo: Endomycorrhiza of unidentified type, corresponds to Endo-unidentified in this data base
Endo er.: Endomycorrhiza of ericoid type, corresponds to Ericoid mycorrhiza in modern terminology
Ph.th.end.: Zygomycetic tamniskophagic endomycorrhiza, corresponds to AM in modern terminology
Ps.end.: Pseudo-endomycorrhiza, corresponds to DS in modern terminology
VAM: Vesicular-arbuscular mycorrhiza, corresponds to AM in modern terminology
Modern term for type of mycorrhiza: modern term for type of mycorrhiza according to (Smith S.E. 2009). This field can have any of the following categories:
AM: arbuscular mycorrhiza
Arbutoid: arbutoid mycorrhiza
Ectomycorrhiza: ectomycorrhiza
Endo-unidentified: non-parasitic fungi found in the root tissue. However they do not create any known type of endo-mycorrhiza
Ericoid: ericoid mycorrhiza
DS: dark septate endophyte mycorrhiza
Orchid: orchid mycorrhiza
No: non-mycorrhizal plant
Site number: number of a site where the plant material was collected. The field can have values from 1 to 154. For detailed site description see file sites.txt
Intensity of mycorrhizal infection: value of intensity of mycorrhizal infection measured by the team of Selivanov, ranging from 1 to 100. For the methods to detect the intensity of mycorrhizal infection see under Methods.
Literature data on presence-absence of mycorrhiza: this field is empty for the species-site combinations where the group of Selivanov measured mycorrhizal intensity
"+": indicates that according to the appendix in the thesis of Selivanov (1976) the species was reported in Russian literature as possessing the given type of mycorrhiza at given site
"-": indicates that, according to the appendix in the thesis of Selivanov (1976), in Russian literature the species was reported as non-mycorrhizal at given site
Range of mycorrhizal intensity MIN and Range of mycorrhizal intensity MAX: minimum and maximum values of mycorrhizal intensity measured by Selivanov and his team (when available)
Taxonomy notes: notes on possible inconsistency sources in vascular plant taxonomy
Description of sampling sites
A. Data Set File
Identity: Sites.csv
Size: 34000 bytes
Format and storage mode: ASCII text, comma separated
B. Header information
Site No.: Unique number of a site of data collection
Climatic zone number: Number of a climatic zone where the site is located, corresponds to the map in Fig. 2
Climatic zone name: Name of a climatic zone where the site is located, corresponds to the map in Fig. 2
Geography: Short verbal description of the site geographical location
Geography details: Detailed verbal description of the site geographical location
Latitude N: Site latitude coordinate (decimal degrees)
Longitude E: Site longitude coordinate (decimal degrees)
Vegetation type: Short description of the vegetation type on the site
Dominant plant species: List of plant species dominant on the site
Soils: Soil type on the site, according to FAO (2003)
Tree species composition: % projected cover of dominant trees on the site
Canopy height: Height of the canopy on the site in cm
Plant cover %: Available data on projected plant cover of the site
Mean tree age: Average age in years of dominant tree species on the site. For the sites situated in mountain forest belts this data is not available. Sites for which the tree age has been set at 0 are not covered by forest.
Forest productivity class: An indication of productivity for forested sites. Range is 1–5; 1 = highest; 5 = lowest. For the sites situated in mountain forest belts this data is not available. Sites for which the productivity is 0 are not covered by forest.
This work was funded by Dutch Organisation for Scientific Research (NWO), grant 047.018.003, and the Russian Foundation for Basic Research (RFBR, grants 08-04-00344, 08-04-92890, 10-04-00780). We would like to thank Mikhail Makarov for consultations on site soil data and Mikhail Kozhin for help with site map creation. Our thanks to many participants of the Teberda expedition of Moscow State University, to the staff of Abisko scientific research station for logistic support and to former staff and assistants of Professor Selivanov for field and laboratory analyses.
Auge, R. M. 2001. Water relations, drought and vesicular-arbuscular mycorrhizal symbiosis. Mycorrhiza 11:3–42.
Becklin, K. M. and C. Galen. 2009. Intra- and Interspecific Variation in Mycorrhizal Associations across a Heterogeneous Habitat Gradient in Alpine Plant Communities. Arctic Antarctic and Alpine Research 41:183–190.
Borowicz, V. A. 2001. Do arbuscular mycorrhizal fungi alter plant-pathogen relations? Ecology 82:3057–3068.
Brundrett, M. 1991. Mycorrhizas in natural ecosystems. Advances in Ecological Research 21:171–313.
Brundrett, M. C. 2009. Mycorrhizal associations and other means of nutrition of vascular plants: understanding the global diversity of host plants by resolving conflicting information and developing reliable means of diagnosis. Plant and Soil 320:37–77.
FAO. 2003. World reference base for soil resources N 103. 2 edition. FAO, Rome.
Gadd, G. M. 1993. Interactions of fungi with toxic metals. New Phytologist 124:25–60.
Giovannetti, M. and B. Mosse. 1980. Evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytologist 84:489–500.
Grunwald, S., J. A. Thompson, and J. L. Boettinger. 2011. Digital Soil Mapping and Modeling at Continental Scales: Finding Solutions for Global Issues. Soil Science Society of America Journal 75:1201–1213.
Kattge, J. and S. Diaz and S. Lavorel and C. Prentice and P. Leadley and G. Bonisch and E. Garnier and M. Westoby and P. B. Reich and I. J. Wright and J. H. C. Cornelissen and C. Violle and S. P. Harrison and P. M. van Bodegom and M. Reichstein and B. J. Enquist and N. A. Soudzilovskaia and D. D. Ackerly and M. Anand and O. Atkin and M. Bahn and T. R. Baker and D. Baldocchi and R. Bekker and C. C. Blanco and B. Blonder and W. J. Bond and R. Bradstock and D. E. Bunker and F. Casanoves and J. Cavender-Bares and J. Q. Chambers and F. S. Chapin and J. Chave and D. Coomes and W. K. Cornwell and J. M. Craine and B. H. Dobrin and L. Duarte and W. Durka and J. Elser and G. Esser and M. Estiarte and W. F. Fagan and J. Fang and F. Fernandez-Mendez and A. Fidelis and B. Finegan and O. Flores and H. Ford and D. Frank and G. T. Freschet and N. M. Fyllas and R. V. Gallagher and W. A. Green and A. G. Gutierrez and T. Hickler and S. I. Higgins and J. G. Hodgson and A. Jalili and S. Jansen and C. A. Joly and A. J. Kerkhoff and D. Kirkup and K. Kitajima and M. Kleyer and S. Klotz and J. M. H. Knops and K. Kramer and I. Kuhn and H. Kurokawa and D. Laughlin and T. D. Lee and M. Leishman and F. Lens and T. Lenz and S. L. Lewis and J. Lloyd and J. Llusia and F. Louault and S. Ma and M. D. Mahecha and P. Manning and T. Massad and B. E. Medlyn and J. Messier and A. T. Moles and S. C. Muller and K. Nadrowski and S. Naeem and U. Niinemets and S. Nollert and A. Nuske and R. Ogaya and J. Oleksyn and V. G. Onipchenko and Y. Onoda and J. Ordonez and G. Overbeck and W. A. Ozinga and S. Patino and S. Paula and J. G. Pausas and J. Penuelas and O. L. Phillips and V. Pillar and H. Poorter and L. Poorter and P. Poschlod and A. Prinzing and R. Proulx and A. Rammig and S. Reinsch and B. Reu and L. Sack and B. Salgado-Negre and J. Sardans and S. Shiodera and B. Shipley and A. Siefert and E. Sosinski and J. F. Soussana and E. Swaine and N. Swenson and K. Thompson and P. Thornton and M. Waldram and E. Weiher and M. White and S. White and S. J. Wright and B. Yguel and S. Zaehle and A. E. Zanne and C. Wirth. 2011. TRY - a global database of plant traits. Global Change Biology 17:2905–2935.
McGonigle, T. P., M. H. Miller, D. G. Evans, G. L. Fairchild, and J. A. Swan. 1990. A new method which gives an objective-measure of colonization of roots by vesicular arbuscular mycorrhizal fungi. New Phytologist 115:495–501.
Meharg, A. A. 2003. The mechanistic basis of interactions between mycorrhizal associations and toxic metal cations. Mycological Research 107:1253–1265.
Muthukumar, T. and K. Udaiyan. 2000. Arbuscular mycorrhizas of plants growing in the Western Ghats region, Southern India. Mycorrhiza 9:297–313.
Newsham, K. K., A. H. Fitter, and A. R. Watkinson. 1995. Multi-functionality and biodiversity in arbuscular mycorrhizas. Trends in Ecology & Evolution 10:407–411.
Postma, J. W. M., P. A. Olsson, and U. Falkengren-Grerup. 2007. Root colonisation by arbuscular mycorrhizal, fine endophytic and dark septate fungi across a pH gradient in acid beech forests. Soil Biology & Biochemistry 39:400–408.
Read, D. J. 1991. Mycorrhizas in ecosystems. Experientia 47:376–391.
Read, D. J. and K. Haselwandter. 1981. Observations on the mycorrhizal status of some alpine plant communities. New Phytologist 88:341–352.
Selivanov, I. A. 1976. Appendix to the Doctoral Thesis "Mycosymbiotrophy as a form of consortic relationships in vegetation of the Soviet Union. [Mikosimbiotrofizm kak forma konsortivnykh svyazei v rastitel'nom pokrove Sovetskogo Soyuza]", in Russian. Perm University, Perm.
Selivanov, I. A. 1981. Mycosymbiotrophy as a form of consortic relationships in vegetation of the Soviet Union. [Mikosimbiotrofizm kak forma konsortivnykh svyazei v rastitel'nom pokrove Sovetskogo Soyuza], in Russian. Nauka, Moscow.
Smith S.E., R. D. J., Smith S. 2009. Mycorrhizal Symbiosis. Academic Press, London.
Smith, S. E. and D. J. Read. 2009. Mycorrhizal Symbiosis. Academic Press, London.
Sokal, R. R. and F. J. Rolf. 1994. Biometry.
Stevens. 2008. Angiosperm Phylogeny Website. Version 9, June 2008. http://www.mobot.org/MOBOT/research/APweb/.
The Plant List. 2010. The Plant List http://www.theplantlist.org/.
Treseder, K. K. and A. Cross. 2006. Global distributions of arbuscular mycorrhizal fungi. Ecosystems 9:305–316.
van der Heijden, M. G. A. and I. A. Sanders, editors. 2002. Mycorrhizal Ecology. Springer, Berlin.
Wang, B. and Y. L. Qiu. 2006. Phylogenetic distribution and evolution of mycorrhizas in land plants. Mycorrhiza 16:299–363.
Wang, Y. P., R. M. Law, and B. Pak. 2010. A global model of carbon, nitrogen and phosphorus cycles for the terrestrial biosphere. Biogeosciences 7:2261–2282.
[Back to E093-059]




