Appendix A. Laboratory calibration of the relationship between heart rate and metabolic rate in Great Cormorants.
Materials and Methods
Great Cormorants Phalacrocorax carbo of approximately 4–6 weeks of age were collected under permit from nests at a mixed P. c. carbo and P. c. sinensis colony at Rutland Water Nature Reserve, UK, in June-July 2004 and 2005. They were immediately transported to the School of Biosciences at The University of Birmingham where they were housed for four months in an indoor facility that included water baths. They were maintained on a hand-delivered diet of defrosted sprats, Sprattus sprattus, with a daily vitamin supplement (fish eater tablets, Mazuri Zoo Foods, Essex, England). When approximately 5–6 months old, the birds were transferred to a 130 m2 outdoor aviary that included a 50 cm deep pond. Birds continued to be maintained on a diet of sprat in the outdoor aviaries, which included the daily vitamin supplement. At the time of measurement the cormorants were well accustomed to handling and experimentation, having previously participated in studies of locomotory energetics (Wilson et al. 2006, White et al. 2008b), behaviour (White et al. 2008c) and vision (White et al. 2007, Martin et al. 2008). Of the 10 birds collected in 2004, six were identified on the basis of gular pouch angle (Newson et al. 2004) as P. c. carbo, two were identified as P. c. sinensis, and the remaining two could not be identified to subspecies, presumably a consequence of hybridisation between P. c. carbo and P. c. sinensis at Rutland Water (Goostrey et al. 1998).
Measurement protocol
Rates of O2 consumption (VO2) and CO2 production (VCO2) of 11 cormorants (mean mass ± SEM: 2.1 ± 0.1 kg) were measured both at rest and during treadmill and swim flume exercise using standard open-flow respirometry (Withers 2001) according to techniques described in detail elsewhere (Wilson et al. 2006, White et al. 2008b) (see Fig. A1 for an example of heart rate and VO2 data). Briefly, room air was drawn by a piston pump (Rietschle Thomas 2688CHI44, Hants, UK) through a clear acrylic chamber which surrounded the bird while on the treadmill (volume 210 l) or swim flume (~225 l, depending on water level). The flow rate through the chamber was 70–80 l min-1, measured using nitrogen dilutions (Fedak et al. 1981), monitored with 2 mechanical flowmeters (KDG 1100 series, 0-40 L min-1, KDG instruments, Sussex, England), and corrected to standard temperature and pressure, dry (STPD). Good mixing within the chamber was assured by the inclusion of three–six 12 × 12 cm fans. A sub-sample of the excurrent airflow was drawn off downstream of the flow meters and passed through a column of indicating DrieriteTM (Hammond Drierite Co, Xenia, Ohio, USA) and an Oxzilla differential O2 analyser (Sable Systems, Las Vegas, Nevada, USA) and ML206 CO2 analyser (ADInstruments, Australia) calibrated with custom gas mixtures provided by a Wösthoff gas mixing pump (type 2M301/a-F, Bochum, Germany) or a butane burner (Withers 2001). Temperature within the chamber and water temperature were measured using a Wheatstone bridge incorporating negative temperature coefficient thermistors supplied with 5V from the digital outputs of a PowerLab ML880 (ADInstruments, Australia). The thermistors were calibrated using a Grant GR150 precision water bath.
Heart rate (fH) was measured using either a Polar T40 non-coded transmitter modified to incorporate subcutaneous electrodes and a Polar OEM Receiver or an implanted heart rate data logger of the same type used in the free-living birds (see main text). The Polar transmitter generates an electromagnetic pulse corresponding with the R-wave of the electrocardiogram (ECG). Upon receiving this transmission, the receiver generates a 1 ms 3V pulse. The implanted data loggers incorporated a low-power radio frequency transmitter which emitted a short VHF pulse on each QRS wave of the ECG. The radio pulse was detected and converted to an analogue voltage output by a radio receiver.
The voltage outputs of the gas analysers, thermistors, and Polar or radio receiver were recorded at a sampling frequency of 200 Hz (for the Polar system) or 1000 Hz (for the implanted loggers) by a PowerLab ML880 A/D converter and Chart software (ADInstruments, Australia).
Birds were exercised on the treadmill or swim flume at a range of speeds (treadmill: 0.5 – 1.5 m s-1; flume: 0.2 – 1.1 m s-1) and temperatures (air: 4.4 – 26.4 ºC; water: 3.2 – 26.3 ºC). The speed range spanned the lowest treadmill or flume speed available and the highest speed at which the birds would maintain station within the respirometer. Either prior to or at the completion of the exercise protocol, birds were allowed to rest until VO2 and VCO2 were low and stable (45 min – 7 h for resting measurements on the treadmill during the day, 4.5 – 11 h for basal measurements at night; most birds would not rest on the flume) and resting VO2 and VCO2 were calculated as the average over a 5 min period. Some birds were placed within the respirometer chamber on the evening before the exercise protocol so that resting metabolic rate could be measured during both the inactive (night) circadian phase prior to exercise, as well as during the active (day) circadian phase. For inactive phase measurements, birds were placed in the chamber before midnight, and resting VO2 data were taken at least 4.5 hours later. Birds were left undisturbed and the respriometer chamber was covered for all measurements of resting metabolic rate. Birds always stood during measurements of resting metabolic rate. Birds were also observed to stand almost all of the time in the outdoor aviaries. ‘Instantaneous’ correction of VO2 and VCO2 was undertaken using the Seymour et al. modification of the Bartholomew et al. (1981) z-transform method, and VO2 and VCO2 during exercise were calculated as the average of instantaneous VO2 and VCO2 for a 2 min period when both were stable. Washout characteristics of the flume respirometer were such that it was not possible to identify dives individually, so VO2 and VCO2 during diving on the flume were also calculated using 2 min averages.
Data analyses
The effects of a range of factors (speed during treadmill locomotion, surface swimming, and diving; water temperature on the flume, air temperature on the treadmill; body mass; and season during rest on the treadmill) on VO2 during rest and activity on the treadmill and swim flume were assessed using mixed model ANOVA with individual identity as a random factor and all others as fixed factors, with a set at 0.05 for all tests.
To account for variation between individuals in the relationship between fH and VO2 associated with body mass (Nagy 2005, McKechnie et al. 2006, Portugal et al. 2007), VO2 data were rendered mass-independent (cVO2). To do this we determined a scaling exponent (b, where VO2 is proportional to massb) for use in all subsequent analyses that minimised inter-individual differences in VO2. The value of b was selected by trialling a range of values from 0 to 1.5, in mixed model ANOVAs where cVO2 (= VO2 × M-b) was modelled as a function of fH (covariate) and bird identity (random factor). Data for resting and exercise VO2 were included in these analyses, from which it was possible to calculate the component of the standard error of the estimate (SEE) of these predictive relationships attributable to inter-individual variation (Green et al. 2001). A scaling exponent of 1.1, which is shallower than the intraspecific exponent observed for kestrels Falco tinnunculus (Daan et al. 1989), similar to that of macaroni penguins Eudyptes chrysolophus (Green et al. 2001), and broadly congruent with the values observed for developing birds (see Appendix 5 of Glazier 2005), minimised inter-individual error. It was not considered necessary to account for size-dependent changes in fH because even though fH during rest and activity were similar between captive and free-ranging individuals in the present study despite their differences in M (Fig. A4). Thus, assuming that the relationship between M and VO2 established for the captive birds (M: 1.6 to 3.1 kg) holds for the free-living birds (mean mass 3.5 kg, range 3.3–3.7 kg), estimating metabolic rate of the free-living birds using a relationship between fH and cVO2 should account for size-related differences in the fH-VO2 relationship.
The aim of the laboratory experiments was to establish a calibration relationship between fH and cVO2 so that field measurements of fH could be converted into cVO2 and metabolic rate. As recommended by Butler et al. (2004), we endeavoured to ensure that the fH-cVO2 data incorporated in the relationship were obtained under conditions that mimicked field conditions as closely as possible. Thus, since VO2 during diving, swimming on stationary water, and surface swimming was related to water temperature (see results), and preliminary analyses revealed that the relationship between fH and cVO2 was also affected by water temperature, only values of fH and cVO2 measured at the lowest temperatures available (< 10 °C) were included in the analysis of the fH-cVO2 relationship since free-living Great Cormorants from Greenland are unlikely to experience water temperatures above 10 °C (Grémillet et al. 2001, White et al. 2008a). A GLM could be constructed to calculate cVO2 based on measurements of fH and water temperature. However, this would depend on specific knowledge of the water temperatures frequented by the free-living birds, which were not available.
Differences between activity states in the relationship between fH and cVO2 have been identified in a number of species including king penguins Aptenodytes patagonicus, little penguins Eudyptula minor, eider ducks Somateria mollissima, bar-headed geese Anser indicus, and barnacle geese Branta leucopsis (Hawkins et al. 2000, Froget et al. 2002, Ward et al. 2002, Green et al. 2008). In the present study, the relationship between fH and cVO2 was significantly different between resting and active animals. As a result, a two-model approach could have been adopted (e.g. Gessaman 1980, Green et al. 2009), which would have minimised the SEE of cVO2 in the free-living birds (Fig. A5). However, this approach would have demanded that all heart rates recorded in free-living animals be assigned correctly as active or inactive. Since there was no way unambiguously to classify the activity status of the wild birds independent of fH (e.g. Pelletier et al. 2007)a two-model approach was rejected, and a single model approach was adopted. This approach predicted values of cVO2 that were similar to those of the two-model approach (Fig. A5). While the SEEs of these estimates were greater, they were still very much within acceptable limits (CV approximately 10%, Fig. A5).
Results and Discussion
Both mass-independent rate of oxygen consumption (cVO2) and heart rate (fH) varied between the different activity states (Table A1). As might be expected, both cVO2 and fH were at a minimum while the birds rested on the treadmill. Walking on the treadmill induced very large and immediate increases in both fH and cVO2 Indeed, mean cVO2 while exercising on the treadmill at 0.5 to 1.5 m s-1 was four times that at rest. While on the swim flume, the cormorants were rarely restful. As a result, mean cVO2 and fH while the water was stationary were similar to those recorded while the birds swam against a current at or beneath the surface (Table A1). The one-model approach that best described the calibration relationship between cVO2 and fH was a power curve (Fig. A4).
Resting rate of oxygen consumption varied seasonally in both 2005 and 2007 (F1,44 = 5.79, P = 0.02, Fig. A2), but did not differ between years (F1,48 = 0.31, P = 0.58), did not differ between circadian phases (F1,41 = 0.36, P = 0.55), was not related to air temperature over the range of 4 to 26 °C (F1,44 = 3.69, P = 0.06, Fig. A3), and was not related to body mass (F1,10 = 0.49, P = 0.51). Basal metabolic rate, measured during the night at temperatures between 19.7 and 23.0 °C in April 2005, was 29.2 ± 1.9 [SEM] ml min-1 for 5 birds weighing 2.3 ± 0.2 kg. This value is almost identical to the value of 29.3 ml min-1 reported for similarly sized (2.1 kg) Phalacrocorax auritus (Enstipp et al. 2006), but 12–27% higher than other values reported for cormorants: 24.1 ml min-1 for 1.7 kg P. aristotelis measured in June (Enstipp et al. 2005), 26.0 ml min-1 for 1.6 kg P. aristotelis measured between March and August (Bryant and Furness 1995), and 22.9 ml min-1 for 2.4 kg P. carbo sinensis measured between August and October (Schmid et al. 1995). Some of these differences could be associated with seasonal variation in BMR; the lowest BMR we recorded (27 ml min-1 in May 2007, Fig. A2) is 4–18% higher than that reported in the studies listed above.
The independence of VO2 and air temperature in the present study suggests that the lower critical temperature for cormorants is at least as low as 4 °C, which is close to the predicted value of 7.3 °C for a 2.3 kg seabird resident at 52.5°N (the latitude of Birmingham) (Ellis and Gabrielsen 2002) and somewhat lower than the reported value of ~10 °C for P. auritus (Enstipp et al. 2006). In the present study, cormorants do not show circadian variation in resting VO2, which has also been reported for other seabirds (Ellis and Gabrielsen 2002), including cormorants (Enstipp et al. 2005).
During treadmill locomotion, VO2 increased with speed (F1,51 = 34.2, P < 0.001) and body mass (F1,11 = 11.6, P = 0.006), but was not related to air temperature over the range 18 to 27 °C (F1,50 = 2.3, P = 0.13). VO2 was negatively related to water temperature during both rest (F1,31 = 12.2, P = 0.001, Fig. A3), surface swimming (F1,48 = 41.2, P < 0.001, Fig. A3) and diving (F1,11 = 13.7, P = 0.004, Fig. A3). While surface swimming at 0.2 – 0.8 m s-1 or diving at 0.5 – 1.1 m s-1 on the water flume, VO2 was not related to either body mass (F1,9 = 1.83, P = 0.21; F1,15 = 8.27, P = 0.01; F1,1 = 7.85, P = 0.22, respectively) or water speed (F1,42 = 3.85, P = 0.06; F1,2 = 1.23, P = 0.41, respectively). Similarly, in ducks and penguins VO2 during surface swimming is largely independent of speed up to around 0.5 m s-1 (Prange and Schmidt-Nielsen 1970, Woakes and Butler 1983, Baudinette and Gill 1985) and metabolic rate during sub-surface swimming has previously been shown to be not strongly dependent on speed up to around 1.6 m s-1 (Schmid et al. 1995). In contrast, metabolic rate during surface swimming at constant speed is strongly dependent on speed in Brandt’s cormorants Phalacrocorax penicillatus (Ancel et al. 2000).
TABLE A1. Mean (± SEM) mass-independent rate of oxygen consumption (cVO2) and heart rate (fH) while eight Great Cormorants rested and exercised on a variable speed treadmill and swim flume at a range of temperatures. Some individuals would not undertake all activity-states while on the swim flume.
| Experiment | Activity State |
Temperature (°C) |
cVO2 (ml kg-1.1min-1) |
fH (beats min-1) |
N |
| Treadmill | Resting | 4.4 – 8.5 | 13.3 ± 0.7 | 119 ± 8 | 8 |
| Treadmill | Walking (0.5 – 1.5 m s-1) | 4.9 – 8.8 | 53.1 ± 2.3 | 238 ± 15 | 8 |
| Swim flume | Surface swimming (0 m s-1) | 4.0 – 8.9 | 61.0 ± 7.1 | 312 ± 24 | 5 |
| Swim flume | Surface swimming (0.2 – 0.8 m s-1) | 3.6 – 9.1 | 68.2 ± 7.8 | 329 ± 21 | 5 |
| Swim flume | Sub-surface swimming (0.5 – 1.1 m s-1) | 4.4 – 7.4 | 87.5 ± 3.6 | 316 ± 19 | 4 |
 |
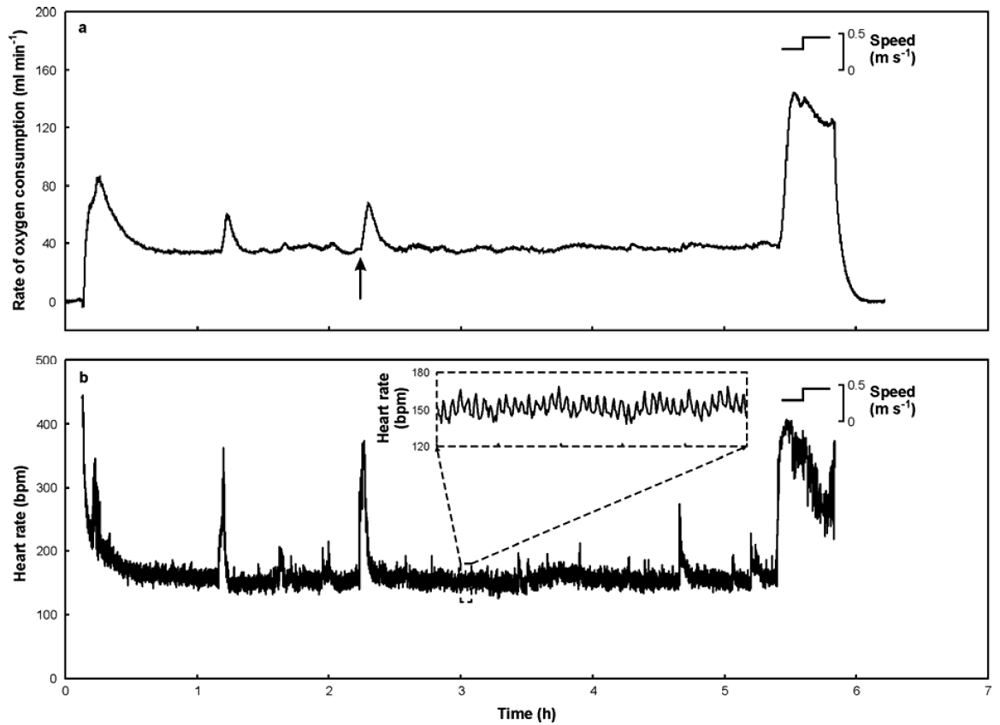
| FIG. A1. Example trace showing (a) rate of oxygen consumption and (b) heart rate of a Great Cormorants (mass 2.1 kg) resting and exercising on a treadmill at a temperature of 6.4 °C. Treadmill speeds are indicated; in this example the cormorant would not walk steadily at the low speed and so those data were disregarded. Arrow indicates a disturbance associated with CRW entering the room. The dashed inset in (b) shows a 5 min period of heart rate data; the cycles in heart rate are caused by respiratory sinus arrhythmia, which is variation in heart rate that occurs as a consequence of variation in vagal tone during a breathing cycle. |
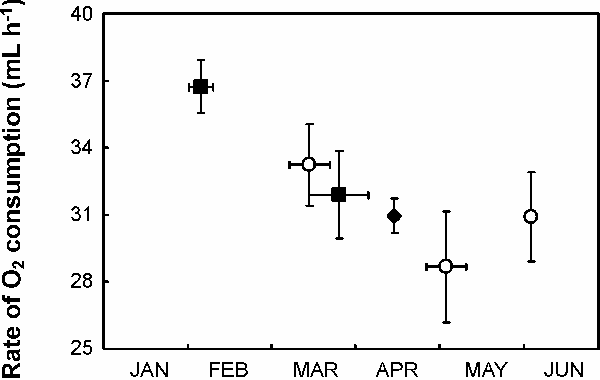 |
| FIG. A2. Seasonal variation in the resting metabolic rate, measured as rate of oxygen consumption, of captive Great Cormorants measured in 2005 (filled symbols) and 2007 (unfilled symbols), either during the day (filled squares, unfilled circles) or night (filled diamond). Dates are shown ± S.D. and rates of oxygen consumption are shown ± SEM; N = 4–8 individuals per data point. |
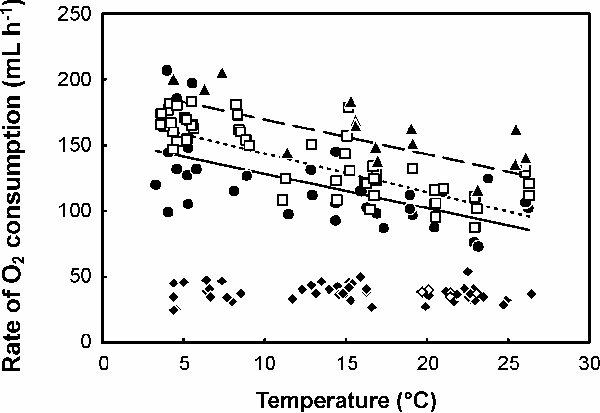 |
| FIG. A3. The effect of temperature (T) on the metabolic rate, measured as rate of oxygen consumption (VO2, mL h-1), of captive Great Cormorants resting in air during the day (filled diamonds) or night (unfilled diamonds), swimming on stationary water (filled circles, solid line), during surface swimming (unfilled squares, dotted line), or during sub-surface swimming (filled triangles, dashed line). For clarity, data are presented adjusted for inter-individual differences in mean rate of oxygen consumption, and data for surface and sub-surface swimming are presented adjusted to a speed of 0.6 m s-1 by accounting for the non-significant positive relationship with swim speed (see text for details). The regressions for swimming on stationary water (VO2 = 154 – 2.6 T), surface swimming (VO2 = 173 – 2.9 T), and sub-surface swimming (VO2 = 196 – 2.7 T) are significant; the relationship between VO2 and T for cormorants resting in air is not significant (see text for details). |
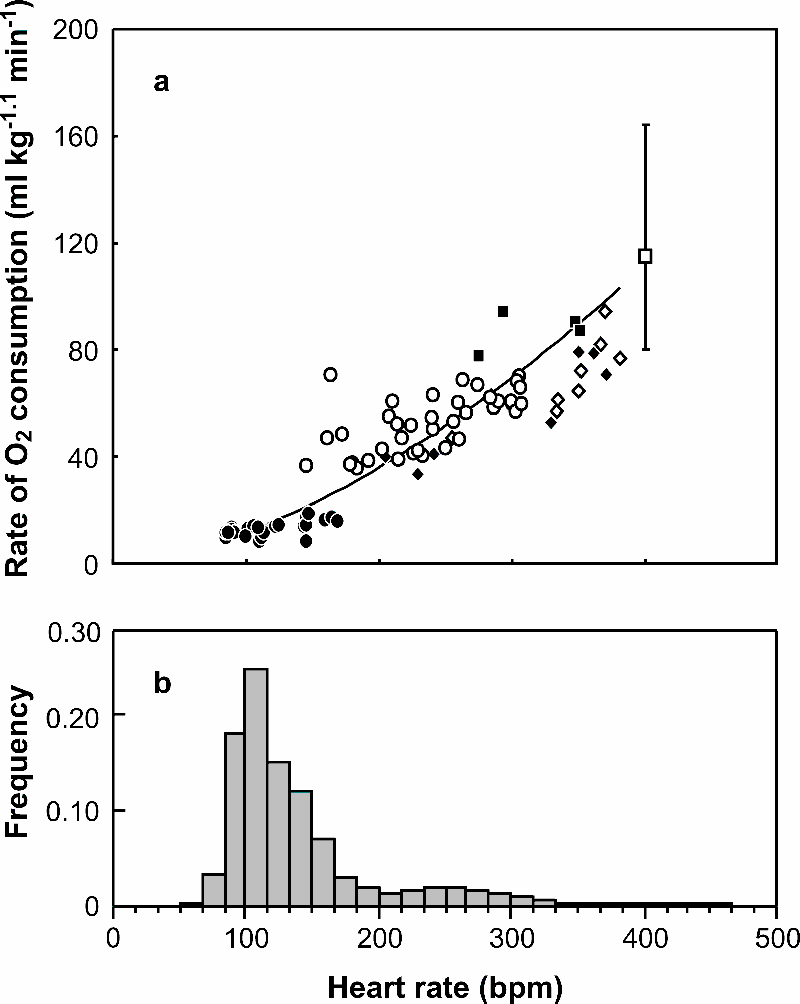 |
| FIG. A4. (a) Calibration relationship (solid line) between heart rate and mass-independent rate of oxygen consumption (cVO2). The equation of the relationship was: cVO2 = 0.0064 fH1.63 (R2 = 89.6, P < 0.001). Data were recorded from eight captive Great Cormorants while they rested (closed circles) and exercised on a treadmill (open circles) at air temperatures below 10 °C, and swam on the surface of stationary water (closed diamonds), swam against a current at the surface (open diamonds), and swam below the surface (closed squares) on a swim flume at water temperatures below 10 °C. Also plotted is cVO2 during flight (± 95% prediction intervals) for free-living Great Cormorants, estimated using the allometric relationship of Bishop et al. (2002), assuming a flight heart rate of approximately 400 beats min-1 (see text). (b) Frequency histogram of heart rates recorded in a free-living Great Cormorant. |
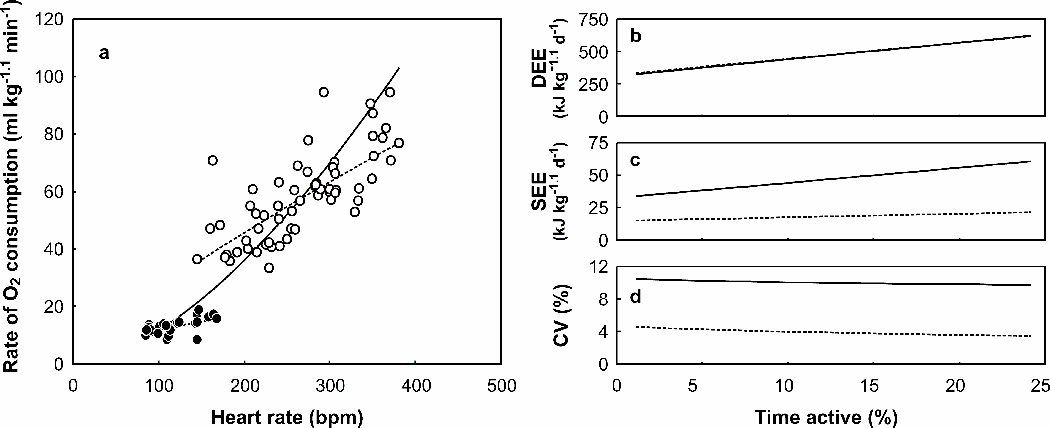 |
| FIG. A5. (a) Comparison of one and two model approaches to derive a predictive relationship between mass-independent rate of oxygen consumption and heart rate in Great Cormorants. The relationship was significantly different when the cormorants were active (open symbols, cVO2 = 0.63 fH0.81) or inactive (closed symbols, cVO2 = 1.19 fH0.50). This two model approach (dashed lines) was compared against a one-model approach (solid line) which ignored this difference. The effect of model selection on the (b) predicted value of mass-independent daily rate of energy expenditure (DEE) (c) the standard error of the estimate (SEE) of DEE and (d) the coefficient of variation (CV = DEE/SEE) was examined by simulating a day in the life of a cormorant. Estimates of DEE by the one- and two-model approaches are similar, so the dashed line is not visible in (b). A day was repeatedly simulated where the proportion of the day spent active was increased sequentially. While active, the cormorant was assumed to spend 10% of its time in flight (heart rate = 400) and 90% of its time on the water (heart rate = 260). The remainder of the time was spent inactive (heart rate = 100). The proportion of time spent active was based on the range found in previous studies of cormorants (studies listed in Table 5) and heart rates selected based on the distribution of found in the present study (Fig. A4). |
LITERATURE CITED
Ancel, A., L. N. Starke, P. J. Ponganis, R. van Dam, and G. L. Kooyman. 2000. Energetics of surface swimming in Brant's cormorants (Phalacrocorax penicillatus Brant). Journal of Experimental Biology 203:3727–3731.
Bartholomew, G. A., D. Vleck, and C. M. Vleck. 1981. Instantaneous measurements of oxygen consumption during pre flight warm-up and post flight cooling in sphingid and saturniid moths. Journal of Experimental Biology 90:17–32.
Baudinette, R. V. and P. Gill. 1985. The energetics of ‘flying’ and ‘paddling’ in water: locomotion in penguins and ducks. Journal of Comparative Physiology B 155:373–380.
Bishop, C. M., S. Ward, A. J. Woakes, and P. J. Butler. 2002. The energetics of barnacle geese (Branta leucopsis) flying in captive and wild conditions. Comparative Biochemistry and Physiology A 133:225–237.
Bryant, D. M. and R. W. Furness. 1995. Basal metabolic rates of North Atlantic seabirds. Ibis 137:219–226.
Butler, P. J., J. A. Green, I. L. Boyd, and J. R. Speakman. 2004. Measuring metabolic rate in the field: the pros and cons of the doubly labelled water and heart rate methods. Functional Ecology 18:168–183.
Daan, S., D. Masman, A. Strijkstra, and S. Verhulst. 1989. Intraspecific allometry of basal metabolic rate: relations with body size, temperature, composition, and circadian phase in the kestrel, Falco tinnunculus. Journal of Biological Rhythms 4:267–283.
Ellis, H. I. and G. W. Gabrielsen. 2002. Energetics of free-ranging seabirds. Pages 359–407 in E. A. Schreiber and J. Burger, editors. The biology of marine birds. CRC press, Boca Raton.
Enstipp, M. R., D. Grémillet, and D. R. Jones. 2006. The effects of depth, temperature and food ingestion on the foraging energetics of a diving endotherm, the double-crested cormorant (Phalacrocorax auritus). Journal of Experimental Biology 209:845–859.
Enstipp, M. R., D. Grémillet, and S.-H. Lorentsen. 2005. Energetic costs of diving and thermal status in European shags (Phalacrocorax aristotelis). Journal of Experimental Biology 208:3451–3461.
Fedak, M. A., L. Rome, and H. J. Seeherman. 1981. One-step N2-dilution technique for calibrating open-circuit measuring systems. Journal of Applied Physiology 51:772–776.
Gessaman, J. A. 1980. An evaluation of heart rate as an indirect measure of daily energy metabolism of the American Kestrel. Comparative Biochemistry and Physiology A 65:273–289.
Glazier, D. S. 2005. Beyond the ‘3/4-power law’: variation in the intra- and interspecific scaling of metabolic rate in animals. Biological Reviews 80:1–52.
Goostrey, A., D. N. Carss, L. R. Noble, and S. B. Piertney. 1998. Population introgression and differentiation in the Great Cormorant Phalacrocorax carbo in Europe. Molecular Ecology 7:329–338.
Green, J. A., P. J. Butler, A. J. Woakes, I. L. Boyd, and R. L. Holder. 2001. Heart rate and rate of oxygen consumption of expercising macaroni penguins. Journal of Experimental Biology 204:673–684.
Green, J. A., L. G. Halsey, R. P. Wilson, and P. B. Frappell. 2009. Estimating energy expenditure of animals using the accelerometry technique: activity, inactivity and comparison with the heart-rate technique. Journal of Experimental Biology 212:471–482.
Green, J. A., C. R. White, and P. J. Butler. 2005. Allometric estimation of metabolic rate from heart rate in penguins. Comparative Biochemistry and Physiology A 142:478–484.
Grémillet, D., S. Wanless, D. N. Carss, D. Linton, M. P. Harris, J. R. Speakman, and Y. Le Maho. 2001. Foraging energetics of arctic cormorants and the evolution of diving birds. Ecology Letters 4:180–184.
Martin, G. R., C. R. White, and P. J. Butler. 2008. Vision and the foraging technique of Great Cormorants Phalacrocorax carbo: pursuit or close-quarter foraging? Ibis 150:485–494.
McKechnie, A. E., R. P. Freckleton, and W. Jetz. 2006. Phenotypic plasticity in the scaling of avian basal metabolic rate. Proceedings of the Royal Society B 273:931–937.
Nagy, K. A. 2005. Field metabolic rate and body size. Journal of Experimental Biology 208:1621–1625.
Newson, S. E., B. Hughes, I. C. Russell, G. R. Ekins, and R. M. Sellers. 2004. Sub-specific differentiation and distribution of Great Cormorants Phalacrocorax carbo in Europe. Ardea 92:3–10.
Pelletier, D., M. Guillemette, J.-M. Grandbois, and P. J. Butler. 2007. It is time to move: linking flight and foraging behaviour in a diving bird. Biology Letters 3:357–359.
Portugal, S. J., J. A. Green, and P. J. Butler. 2007. Annual changes in body mass and resting metabolism in captive barnacle geese (Branta leucopsis): the importance of wing moult. Journal of Experimental Biology 210:1391–1397.
Prange, H. D. and K. Schmidt-Nielsen. 1970. The metabolic cost of swimming in ducks. Journal of Experimental Biology 53:763–777.
Schmid, D., D. Grémillet, and B. M. Culik. 1995. Energetics of underwater swimming in the Great Cormorant (Phalacrocorax carbo sinensis). Marine Biology 123:875–881.
Seymour, R. S., P. C. Withers, and W. W. Weathers. 1998. Energetics of burrowing, running, and free-living in the Namib desert golden mole (Eremitalpa namibensis). Journal of Zoology 244:107–117.
White, C. R., P. J. Butler, D. Grémillet, and G. R. Martin. 2008a. Behavioural strategies of predation in cormorants Phalacrocoracidae foraging under challenging light conditions. Ibis 150 (Special Issue 1):231–239.
White, C. R., N. Day, P. J. Butler, and G. R. Martin. 2007. Vision and foraging in cormorants: more like herons than hawks. PLoS ONE 2:e639. doi:610.1371/journal.pone.0000639.
White, C. R., G. R. Martin, and P. J. Butler. 2008b. Pedestrian locomotion energetics and gait characteristics of a diving bird, the Great Cormorant, Phalacrocorax carbo. Journal of Comparative Physiology B 178:745–754.
White, C. R., G. R. Martin, and P. J. Butler. 2008c. Wing-spreading, wing-drying and food-warming in Great Cormorants Phalacrocorax carbo. Journal of Avian Biology 39:576–578.
Wilson, R. P., C. R. White, F. Quintana, L. G. Halsey, N. Liebsch, G. R. Martin, and P. J. Butler. 2006. Moving towards acceleration for estimates of activity-specific metabolic rate in free-living animals: the case of the cormorant. Journal of Animal Ecology 75:1081–1090.
Withers, P. C. 2001. Design, calibration and calculation for flow-through respirometry systems. Australian Journal of Zoology 49:445–461.
Woakes, A. J. and P. J. Butler. 1983. Swimming and diving in tufted ducks, Aythya fuligula, with particular reference to heart rand gas exchange. Journal of Experimental Biology 107:311–329.