Peter S. Petraitis, Harrison Liu, and Erika C. Rhile. 2009. Barnacle, fucoid, and mussel recruitment in the Gulf of Maine, USA, from 1997 to 2007. Ecology 90:571.
Abstract: Experimental clearings in macroalgal (Ascophyllum nodosum) stands were made at 12 sites in 1996 to determine if mussel beds and macroalgal stands on sheltered intertidal shores of New England represent alternative community states. Sites were located on Swan’s Island, Maine, USA. At each site an uncleared control plot and four sizes of circular clearings, which mimicked ice scour events, were established. The purpose of this data set is to provide access to recruitment data collected in the experimental plots from 1997 to 2007.
Key words: Alternative stable states; barnacles; community ecology; Gulf of Maine; LTREB data; mussels; recruitment; rocky intertidal shores; seaweeds.
Queries regarding the data set can be directed to: [email protected]
INTRODUCTION
Sheltered intertidal shores in the Gulf of Maine, USA, tend to be dominated by either stands of the canopy-forming fucoid seaweeds, (Ascophyllum nodosum and Fucus vesiculosus) or by mixed beds of barnacles (Semibalanus balanoides) and mussels (Mytilus edulis), and Petraitis and Latham (1999) hypothesized that these mussel beds and seaweed stands in sheltered bays could be alternative community states. Petraitis and Latham (1999) also suggested that infrequent ice scour events could initiate the formation of these two communities in sheltered bays by opening up new clearings and allowing divergent successional events to pave the way for alternative community states. This is most likely to occur in large clearings where much of a patch would lie beyond the edge effects of the surrounding community, and thus giving recruits, which arrive in opportunistic fashion, sufficient time and space to become established and develop into either a mussel bed or a seaweed stand. Once started, each assemblage would be maintained by positive feedbacks and ecosystem engineering within the community (Jones et al. 1994). Based on this scenario, Petraitis and Latham (1999) predicted succession should be not only divergent but also more variable in large clearings than in small clearings. In contrast, if mussel beds and fucoid stands were the result of site-specific differences in consumer control then clearing size should have no effect on the patterns of succession (Menge 1976, Bertness et al. 2002).
Experimental clearings of different sizes were established in A. nodosum stands in 1996 to test the hypothesis that mussel beds and fucoid stands were alternative community states. Clearings were made at 12 sites in sheltered bays on Swan’s Island, Maine. The set of clearings at each site included four clearings (1, 2, 4, and 8 m in diameter), and a control plot that was not cleared for a total of 60 plots (Petraitis and Dudgeon 1999). Clearings were made by scraping the surface with paint scrapers to mimic the effects of ice scour (see Dudgeon and Petraitis 2001 for details). Scraping removed A. nodosum holdfasts, barnacles, mussels, and other organisms and was similar in effect to a severe ice scour event, which completely removes everything from the surface.
If fucoid stands and mussel beds are alternative community states in sheltered bays, then some of the large clearings should diverge from their original state prior to the disturbance (i.e., A. nodosum stands) and become mussel beds. Recruitment, which helps drive successional changes, should also more variable and divergent in larger clearings. Analyses based on the recruitment data archived here shows that recruitment patterns of fucoids, barnacles, and mussels are indeed more variable in larger clearings and show overall differences based on clearing size (Dudgeon and Petraitis 2001, Petraitis and Methratta 2006, Methratta and Petraitis, in press). Data on densities and percentage cover of the most common marine organisms in these plots are also archived (Petraitis and Vidargas 2006, Petraitis et al. 2008), and analyses show that the pattern of succession depends on clearing size and not on site-specific differences (Petraitis et al. 2003, Petraitis and Dudgeon 2005).
Others claimed to have duplicated Petraitis’ experimental design and to have failed to find evidence of alternative community states (Bertness et al. 2002, 2004a, Bertness et al. 2004b). However, these experiments in fact did not use Petraitis’ experimental design and did not meet the criteria for testing for alternative community states (Petraitis and Dudgeon 2004b). The critical test has several distinct requirements (Connell and Sousa 1983, Peterson 1984, Sousa and Connell 1985, Petraitis and Dudgeon 2004a, Suding et al. 2004, Schröder et al. 2005). First, experimental manipulations must show that the same site could be occupied by different self-replacing communities (Peterson 1984). In addition, the manipulations must be only pulse perturbations that mimic a natural event in spatial extent, temporal duration and its effect on species in the system (Connell and Sousa 1983). While at first glance, these four criteria – same site, different communities, self-replacement, and natural pulse perturbations – are unambiguous, there have been few experimental tests that met all criteria. Bertness et al.’s (2002, 2004b) experiments included two types of sites (mussel beds and fucoid stands) and used both press and pulse perturbations (caging and scraping), and in so doing, they failed to meet the requirements of using one type of site and only natural pulse perturbations (Petraitis and Dudgeon 2004b). The data archived here and elsewhere (Petraitis and Vidargas 2006, Petraitis et al. 2008) represent one of the longest and best replicated experiments testing the theory of multiple stable states using only pulse perturbations that mimic a natural event and carried out in a single habitat type.
METADATA
CLASS I. DATA SET DESCRIPTORS
A. Data set identity: Recruitment Data from Experimental Plots on Sheltered Intertidal Shores in the Gulf of Maine 1997–2007.
B. Data set identification code: Recruitment_data_97-07.txt
C. Data set description
Principal Investigator: Peter S. Petraitis, Department of Biology, University of Pennsylvania, Philadelphia, PA 19104-6018 USA
Abstract:Experimental clearings in macroalgal (Ascophyllum nodosum) stands were made at 12 sites in 1996 to determine if mussel beds and macroalgal stands on sheltered intertidal shores of New England represent alternative community states. Sites were located on Swan’s Island, Maine, USA. At each site an uncleared control plot and four sizes of circular clearings, which mimicked ice scour events, were established. The purpose of this data set is to provide access to recruitment data collected in the experimental plots from 1997 to 2007.
D. Key words: Alternative stable states; barnacles; community ecology; Gulf of Maine; LTREB data; mussels; recruitment; rocky intertidal shores; seaweeds.
CLASS II. RESEARCH ORIGIN DESCRIPTORS
A. Overall project description
Identity: Recruitment Data from Experimental Plots on Sheltered Intertidal Shores in the Gulf of Maine 1997–2007
Originator: Peter Petraitis, Department of Biology, University of Pennsylvania, Philadelphia, PA 19104-6018.
Period of Study: 1997 – 2007 (ongoing).
Objectives: To use recruitment patterns in experimental plots to test hypothesis concerning alternative community states.
Abstract: Same as above.
Sources of funding: All data collection has been supported by NSF (OCE 95-29564 and DEB LTREB 03-14980).
B. Specific subproject description
Site description: Experimental clearings were established at 12 replicate sites in four bays on Swan’s Island, Maine, USA with three sites nested in each bay. The bays are Mackerel Cove and Seal Cove, which are on the north side of the island, and Burnt Cove Harbor and Toothacher Cove, which are on the south side of the island. See Dudgeon and Petraitis (2001, Fig. 2) for map with locations of sites and bays, and Petraitis et al. (2008) for latitude and longitude in degrees and decimal minutes of reference bolts in experimental plots.
Site type: The sites are in the mid intertidal zones of sheltered bays, and experimental clearings are between 0.3 and 1.0 m above Mean Low Water.
Geography: Swans Island is located at 44°10' N, 68°25' W in the Gulf of Maine, USA. The island is approximately 36 km2 in area with a highly irregular coastline.
Habitat: The mid intertidal shores are protected from wave surge and dominated by the rockweed A. nodosum.
Geology: The shoreline surfaces at the different sites are a mixture of granite and basalt outcrops, boulder fields, and some muddy patches in the most protected sites.
Watersheds/hydrology: The exposure to waves at these sites ranges from extremely protected to moderately protected.
Site history: N/A
Climate: Coastal northeastern USA
Experimental design: At each site, four circular clearings (1, 2, 4, and 8 m in diameter) and uncleared control plot were created in A. nodosum stands in 1996. It was not possible to make the clearings during the winter because of limited access and dangerous conditions due to snow and ice. Thus plots were originally scraped with paint scrapers between 22 June and 17 August 1996 and then re-scraped between 6 and 10 February 1997 to mimic winter ice events. Two holes (6.35 mm in diameter and 25.4 mm in depth) were drilled in the center of each plot to accommodate plastic anchor sleeves and stainless steel screws, which were used to secure recruitment surfaces. The screws were approximately 40 cm apart. Full description of the design can be found elsewhere (Petraitis and Dudgeon 1999, Dudgeon and Petraitis 2001, Petraitis and Dudgeon 2005, Petraitis and Vidargas 2006).
Design characteristics: The experimental set-up is a partially nested design with clearing size fully crossed with bays and with sites nested within bays. Sites and bays are considered random effects and clearing size is a fixed treatment effect. The design is fully discussed in Petraitis and Dudgeon (2004b).
Sampling methods: Details of recruitment substrates and sampling methods are given in Dudgeon and Petraitis (2001). Counts were carried out by various people over the years and while all counting was overseen by P. S. Petraitis, E. C. Rhile or S. R. Dudgeon, there is danger of observer bias among years. Barnacle counts are the easiest to do and least likely to have a bias. Mussel counts are the most difficult to do; analyses of these data should use log-transformed data to dampen the effects of year to year bias.
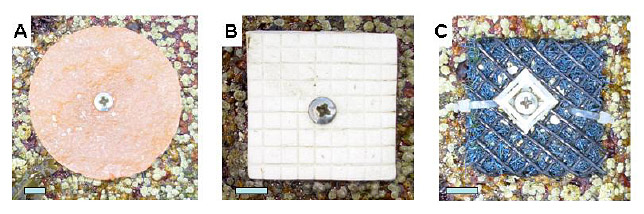
Barnacle recruitment. Plates used for barnacle recruitment were resin castings made from latex molds of natural granite rock (Fig. 1). Plates were disk-shaped; approximately 0.67 cm thick and 7.1 cm in diameter (39.6 cm2 in area). Barnacle recruitment in Maine begins in March and continues through May and so plates for barnacle recruitment were usually placed into the plots in late March (average date: 26 March; range: 1 March – 31 May) and usually collected in late May (average date: 22 May; range: 3 April – 28 June) for an average of 57 days (range: 27–77 days). A complete count of cyprid larvae and newly metamorphosed spat on each plate was done within a day of collection using a dissecting scope at 25×. Data file provides total count per plate.
 |
| FIG. 1 Recruitment surfaces used for barnacles (panel A), fucoids (B), and mussels (C). Blue bars are 1 cm long. |
Fucoid recruitment. White ceramic tiles (approx 4.5 × 4.5 × 0.6 cm) were used for collect recruits of the brown algae Fucus vesiculosus and Ascophyllum nodosum (Fig. 1). Surface of tiles had a grid of grooves that divided the tile into 0.5 × 0.5 cm squares forming a 9 × 9 grid. Grooves were approximately 0.5 mm deep and 0.5 mm wide, and tended to trap developing embryos. A. nodosum recruitment in eastern Maine near Swan’s Island occurs over 3–7 weeks in spring (Bacon and Vadas 1991). F. vesiculosus releases gametes in late April – early May and recruitment peaks in May–June; however individuals remain reproductive until October. For most years, recruits of the two species were lumped together because it is impossible to distinguish embryos and extremely difficult to distinguish young germlings. However, in 1997, we are confident the counts are nearly all A. nodosum for three reasons (Dudgeon and Petraitis 2001). First, F. vesiculosus adults were extremely rare prior to 2002 and so the source for zygotes was very limited. Background percentage cover in 1999 averaged 4.8% excluding the July 4th site (JL), which had 52% F. vesiculosus cover. Second, tiles in 1997 were taken in prior to the peak of F. vesiculosus recruitment. And finally, F. vesciulosus germlings have apical hairs and A. nodosum germlings do not; nearly of the germlings in 1997 lacked apical hairs By 2002, F. vesiculosus had invaded most clearings and formed large beds of mature reproductive individuals (P. S. Petraitis, peronal observation), and most of the recruits after 2002 were F. vesiculosus (Methratta and Petraitis, in press). Tiles were usually placed out in late March (average date: 25 March; range: 1 March – 31 May) and usually collected in late May (average date: 22 May; range: 3 May – 28 June) for an average of 58 days (range: 27–77 days). Collected tiles were kept moist, and the number of embryos and germlings were counted in five 0.5 × 0.5 cm squares (i.e., the flats) and five 0.5 cm grooves (i.e., the grooves) per tile using a dissecting scope. Squares to be counted were randomly chosen; for each square, one of the grooves that bordered the square was then chosen. The total number of recruits per five squares (1.25 cm2 in total area) and per five grooves (1.25 cm in total length) are reported.
Mussel recruitment. Settling mussel larvae tend to attach initially to filamentous algae and so fibrous pads were used as substrates for recruitment. Pads were approximately 5 × 5 cm and cut from furnace filters (range of fiber diameter = 0.3–0.5 mm). Pads were wrapped in a 5 cm × 13 cm piece of plastic mesh (width of mesh opening: 4.6–5.0 mm; diameter 1.5 mm), which was closed with two cable ties (Fig. 1). These pads differ from collectors used by others (e.g., Menge 1992, Leonard et al. 1998). Pads tend to erode during emersion and so they were weighed after collection. On Swan’s Island, mussels begin to recruit in June, peak in July and early August, and continue through September (Bethel 1973, Petraitis 1991). Pads were usually placed out in late May (average date: 27 May; range: 1 May – 27 June) and usually collected in late August (average date: 21 August; range: 8 August – 30 August) for an average of 85 days (range: 67–108 days). Upon collection, pads were preserved in 70% isopropyl alcohol. For counting, pads were torn open and rinsed in water over 425 µm and 300 µm sieves. Pads were then dried and weighed. In most years, mussels trapped on each sieve were rinsed onto a tray with a 2 × 2 cm grid of 32 squares (4 × 8), and mussels were counted in five (1998, 2000, and 2001) or ten (2003–2007) randomly chosen squares. Data were not collected in 1998 and 2002. In 1997, counts were collected as 10 circular fields, 1 cm in diameter (Dudgeon and Petraitis 2001); raw totals were multiplied by 5.092 to give numbers per 40 cm2. In 1999, mussels in the entire tray were counted. Counts in data file are reported as numbers per 40 cm2 and rounded to the nearest whole number (i.e., equivalent to the total of ten 2 × 2 cm squares).
Taxonomy and systematics: Names for fucoid algae follow usage in Taylor (1957) and for barnacles and mussels follow usage in Gosner (1971).
Permit history: Clearings were made under permit from the State of Maine’s Department of Marine Resources.
Legal/organizational requirements: None.
Project personnel: Peter Petraitis, Steve Dudgeon, E. T. Methratta, Erika Carlson Rhile, Nick Vidargas, Harrison Liu
CLASS III. DATA SET STATUS AND ACCESSIBILITY
A. Status
Latest update: The data set spans the period of 1997–2007. Data collection is ongoing through the present and will be added as collected and verified.
Latest Archive date: 20 July 2008
Metadata status: The metadata are complete and up to date.
Data verification: Entries in Excel files were checked for outliers, which were then re-checked against original hand-written entries in the data books.
B. Accessibility
Storage location and medium: (Ecological Society of America data archives [http://esapubs.org/archive/default.htm], URL published in each issue of its journals). Original data books are housed at the University of Pennsylvania; data files exist on author’s personal computer and on CD in MSExcel format.
Contact person: Peter Petraitis, email: [email protected], Tel. 215.898.4207, Department of Biology, University of Pennsylvania, Philadelphia, PA 19104-6018, USA
Copyright restrictions: None.
Proprietary restrictions: None.
Costs: None.
CLASS IV. DATA STRUCTURAL DESCRIPTORS
A. Data Set File
Identity: Recruitment_data_97-07.txt
Size: 660 records, not including header row.
Format and storage mode: ASCII text, tab delimited. No compression scheme was used.
Header information: See variable names in Section B.
Alphanumeric attributes: Mixed.
Special characters/fields: Missing data denoted as -999.9
Authentication procedures: Sums of the numeric columns are used for cross-checking successful downloads of data file. The sums are: Fucoid_F = -118,042.6, Fucoid_G = -108,147.4, Fucoid_T = -44,659.8, Barnacle = 50,957.1, M_300 = -89,566.5, M_425 = -94,863.5, M_WT = -253,109.78.
B. Variable information:
Year: Gives year of sampling; note that the suffixes “a” for spring samples and “b” for summer samples used in Petraitis and Vidargas (2006) have been dropped.
Bay: Names of bays are consistent with names on U.S. Geological Survey 7.5 minute topographic quadrangle for Swan’s Island. Codes for bay names are: BC = Burnt Coat Harbor, MC = Mackerel Cove, SC = Seal Cove, TC = Toothacher Cove.
Site: Site names were assigned based on either names that appear on USGS maps or were named by Petraitis (see Petraitis and Vidargas, 2006 for details of names).
Size: Diameter of experimental clearings. Uncleared control plots are given as 0.
Numeric variables: Variables are counts per area or length or weights in grams.
Date variables: Dates of when recruitment substrates were placed out and taken in are given in month/day/year format. If a substrate was not placed out, both date entries are listed as “nd”. If a substrate was placed out but lost, the initial date is given and the final date is listed as “nd”.
TABLE 1. Summary of variable information. |
|||||
Variable name |
Variable definition |
Units |
Storage type |
Range |
Missing value codes |
Year |
Year in which sampling was done |
N/A |
Character |
N/A |
N/A |
Bay |
Bay in which sampling was done |
N/A |
Character |
N/A |
N/A |
Site |
Site at which sampling was done |
N/A |
Character |
N/A |
N/A |
Size |
Diameter of clearing in meters |
N/A |
Character |
N/A |
N/A |
Fucoid_F |
Number of fucoid zygotes and germlings on a total of five flats |
Number per 1.25 cm2 |
Floating point |
0 – 549 |
-999.9 |
Fucoid_G |
Number of fucoid zygotes and germlings in a total of five grooves |
Number per 1.25 cm |
Floating point |
0 – 949 |
-999.9 |
Fucoid_T |
Sum of Fucoid_F and Fucoid_G |
Number per 1.25 cm2 + number per 1.25 cm |
Floating point |
0 – 1125 |
-999.9 |
Barnacles |
Number of S. balanoides cyprids and metamorphs per plate |
Number per 39.6 cm2 |
Floating point |
0 – 545 |
-999.9 |
M_300 |
Subsample of junvenile M. edulis trapped on 300 um sieve |
Number per 40 cm2 |
Floating point |
0 – 4184 |
-999.9 |
M_425 |
Subsample of juvenile M. edulis trapped on 425 um sieve |
Number per 40 cm2 |
Floating point |
0 – 1264 |
-999.9 |
M_WT |
Dry weight of mussel pad |
Grams |
Floating point |
0.67 – 4.36 |
-999.9 |
F_start |
Date fuciod tiles placed out (Month/Day/Year) |
N/A |
Character |
N/A |
nd |
F_end |
Date fuciod tiles taken in (Month/Day/Year) |
N/A |
Character |
N/A |
nd |
B_start |
Date barnacle plates placed out (Month/Day/Year) |
N/A |
Character |
N/A |
nd |
B_end |
Date barnacle plates taken in (Month/Day/Year) |
N/A |
Character |
N/A |
nd |
M_start |
Date mussel pads placed out (Month/Day/Year) |
N/A |
Character |
N/A |
nd |
M_end |
Date mussel pads taken in (Month/Day/Year) |
N/A |
Character |
N/A |
nd |
CLASS V. SUPPLEMENTAL DESCRIPTORS
A. Data acquisition
Data forms: “Rite in the Rain” field log books.
Location of completed data forms: 330 Leidy Labs, Department of Biology, University of Pennsylvania, Philadelphia, PA 19104-6018, USA
Data entry/verification procedures: Personnel recorded data in log books and data were then entered into MSExcel spreadsheets and double-checked. Files are stored on author’s personal computer and on CD as MSExcel files. Log books are held at author’s address.
B. Quality assurance/quality control procedures: See earlier comments on data entry and verification (Class III, Section A; Class V, Section A).
C. Related material: Data on adult mussel mortality (1996, 1999, 2000, 2003, 2004, and 2007) in the same plots have been collected but have not been archived. Sampling of densities and percentage cover by the common invertebrates and algae in these plots from 1996 to 2007 are available (Petraitis and Vidargas 2006, Petraitis et al. 2008).
D. Computer programs and data processing algorithms: None used; data in file were checked against entries in original data books.
E. Archiving: N/A
F. Publications using the data set:
Dudgeon, S. R., and P. S. Petraitis. 2001. Scale-dependent recruitment and divergence of intertidal communities. Ecology 82:991–1006.
Petraitis, P. S., and E. T. Methratta. 2006. Using patterns of variability to test for multiple community states on rocky intertidal shores. Journal of Experimental Marine Biology and Ecology 338:222–232.
Methratta, E. T., and P. S. Petraitis. In press. Propagation of scale-dependent effects from recruits to adults in barnacles and seaweeds. Ecology.
G. Publications using the same sites:
Dudgeon, S. R., J. E. Kübler, W. A. Wright, R. L. Vadas, Sr., and P. S. Petraitis. 2001. Natural variability in zygote dispersal of Ascophyllum nodosum at small spatial scales. Functional Ecology 15:595–604.
Dudgeon S. R., and P. S. Petraitis. 2005. First year demography of a foundation species, Ascophyllum nodosum, and its community implications. Oikos 109:405–415.
Petraitis, P. S., and S. R. Dudgeon. 1999. Experimental evidence for the origin of alternative communities on rocky intertidal shores. Oikos 84:239–245.
Petraitis, P. S., E. C. Rhile, and S. R. Dudgeon. 2003. Survivorship of juvenile barnacles and mussels: spatial dependence and the origin of alternative communities. Journal of Experimental Marine Biology and Ecology 293:217–236.
Petraitis, P. S., and S. R. Dudgeon. 2005. Divergent succession and implications for alternative states on rocky intertidal shores. Journal of Experimental Marine Biology and Ecology 326:14–26.
H. History of data set usage
Data request history: N/A
Data set update history: N/A
Review history: N/A
Questions and comments from secondary users: N/A
ACKNOWLEDGMENTS
We thank the residents of Swan’s Island who supported this research for providing access to the shore across their properties.Since 2004, Erika Rhile’s students from The High Cheverus School have helped with data collection and entry, and we thank the school for making their involvement possible. Research was supported by National Science Foundation grants (OCE 95-29564 and DEB LTREB 03-14980) to P. S. Petraitis.
LITERATURE CITED
Bacon, L., and R. L. Vadas. 1991. A model for gamete release in Ascophyllum nodosum (Phaeophyta). Journal of Phycology 27:166–173.
Bertness, M. D., G. C. Trussell, P. J. Ewanchuk, and B. R. Silliman. 2002. Do alternate stable community states exist in the Gulf of Maine rocky intertidal zone? Ecology 83:3434–3448.
Bertness, M. D., G. C. Trussell, P. J. Ewanchuk, and B. R. Silliman. 2004a. Do alternate stable community states exist in the Gulf of Maine rocky intertidal zone? Reply. Ecology 85:1165–1167.
Bertness, M. D., G. C. Trussell, P. J. Ewanchuk, B. R. Silliman, and C. M. Crain. 2004b. Consumer-controlled community states on Gulf of Maine rocky shores. Ecology 85:1321–1331.
Bethel, L. J. 1973. Spawning and settlement of Mytilus edulis L. Ph.D dissertation. University of Pennsylvania, Philadelphia, Pennsylvania, USA.
Connell, J. H., and W. P. Sousa. 1983. On the evidence needed to judge ecological stability or persistence. American Naturalist 121:789–824.
Dudgeon, S., and P. S. Petraitis. 2001. Scale-dependent recruitment and divergence of intertidal communities. Ecology 82:991–1006.
Gosner, K. L. 1971. Guide to identification of marine and estuarine invertebrates. John Wiley, New York, New York, USA.
Jones, C. G., J. H. Lawton, and M. Shachak. 1994. Organisms as ecosystem engineers. Oikos 69:373–386.
Leonard, G. H., J. M. Levine, P. R. Schmidt, and M. D. Bertness. 1998. Flow-driven variation in intertidal community structure in a Maine estuary. Ecology 79:1395–1411.
Menge, B. A. 1976. Organization of New England rocky intertidal community - role of predation, competition, and environmental heterogeneity. Ecological Monographs 46:355–393.
Menge, B. A. 1992. Community regulation - under what conditions are bottom-up factors important on rocky shores. Ecology 73:755–765.
Methratta, E. T., and P. S. Petraitis. In press. Propagation of scale-dependent effects from recruits to adults in barnacles and seaweeds. Ecology.
Peterson, C. H. 1984. Does a rigorous criterion for environmental identity preclude the existence of multiple stable points? American Naturalist 124:127–133.
Petraitis, P. S. 1991. Recruitment of the mussel Mytilus edulis on sheltered and exposed shores in Maine, USA. Journal of Experimental Marine Biology and Ecology 147:65–80.
Petraitis, P. S., and S. R. Dudgeon. 1999. Experimental evidence for the origin of alternative communities on rocky intertidal shores. Oikos 84:239–245.
Petraitis, P. S., and S. R. Dudgeon. 2004a. Detection of alternative stable states in marine communities. Journal of Experimental Marine Biology and Ecology 300:343–371.
Petraitis, P. S., and S. R. Dudgeon. 2004b. Do alternate stable community states exist in the Gulf of Maine rocky intertidal zone? Comment. Ecology 85:1160–1165.
Petraitis, P. S., and S. R. Dudgeon. 2005. Divergent succession and implications for alternative states on rocky intertidal shores. Journal of Experimental Marine Biology and Ecology 326:14–26.
Petraitis, P. S., and R. E. Latham. 1999. The importance of scale in testing the origins of alternative community states. Ecology 80:429–442.
Petraitis, P. S., H. Liu, and E. C. Rhile. 2008. Densities and cover data for intertidal organisms in the Gulf of Maine, USA from 2003 to 2007 (Ecological Archives E089-032). Ecology 89:588.
Petraitis, P. S., and E. T. Methratta. 2006. Using patterns of variability to test for multiple community states on rocky intertidal shores. Journal of Experimental Marine Biology and Ecology 338:222–232.
Petraitis, P. S., E. C. Rhile, and S. Dudgeon. 2003. Survivorship of juvenile barnacles and mussels: spatial dependence and the origin of alternative communities. Journal of Experimental Marine Biology and Ecology 293:217–236.
Petraitis, P. S., and N. Vidargas. 2006. Marine intertidal organisms found in experimental clearings on sheltered shores in the Gulf of Maine, USA (Ecological Archives E087-047). Ecology 87:795.
Schröder, A., L. Persson, and A. M. De Roos. 2005. Direct experimental evidence for alternative stable states: a review. Oikos 110:3–19.
Sousa, W. P., and J. H. Connell. 1985. Further comments on the evidence for multiple stable points in natural communities. American Naturalist 125:612–615.
Suding, K. N., K. L. Gross, and G. R. Houseman. 2004. Alternative states and positive feedbacks in restoration ecology. Trends in Ecology & Evolution 19:46–53.
Taylor, W. R. 1957. Marine algae of the northeastern coast of North America., Second edition. The University of Michigan Press, Ann Arbor, Michigan, USA.