Ecological Archives A025-135-A1
Aditya Singh, Shawn P. Serbin, Brenden E. McNeil, Clayton C. Kingdon, and Philip A. Townsend. 2015. Imaging spectroscopy algorithms for mapping canopy foliar chemical and morphological traits and their uncertainties. Ecological Applications 25:2180–2197. http://dx.doi.org/10.1890/14-2098.1
Appendix A. Comparisons of model accuracies with literature, species-wise estimates of canopy foliar traits,comparisons of models built with different canopy weighing schemes, comparisons of spatial predictions of foliar traits.
Table A1. Review of recent literature on the application of imaging spectroscopy to map foliar chemistry. Note that in order to conform to the analytical methods presented in this study, we do not include studies that employ inversion of radiative transfer models to map canopy chemical properties.
Trait |
Vegetation type |
Location |
Sensor |
Plots |
Images |
Method |
R2Cal |
RMSECal |
R2Val |
RMSEVal |
Source |
C |
Mixed needleleaf-broadleaf |
Switzerland |
HyMap |
28 |
4 |
B&B |
0.31 |
1.21 (RMSE) |
Huber et al. (2008) |
||
Lig |
Oak, Pine |
Wisconsin |
AIS |
20 |
1 |
MLR |
0.85 |
1.9 (SE) |
Wessman et al. (1989) |
||
Lig |
Juniper - Coastal rainforest |
West-central Oregon |
AVIRIS |
17 |
4 |
MLR |
0.93 |
277.80 (SEC) |
Johnson et al. (1994) |
||
Lig |
N Hardwood and needleleaf |
Harvard Forest, MA |
AVIRIS |
21 |
1 |
MLR |
0.70 |
2.38 (SECV) |
0.27 |
3.87 (SEP) |
Martin and Aber (1997) |
Lig |
N Hardwood and needleleaf |
Blackhawk Island, WI |
AVIRIS |
20 |
1 |
MLR |
0.90 |
0.85 (SECV) |
0.01 |
4.33 (SEP) |
Martin and Aber (1997) |
Lig:N |
N Hardwood and needleleaf |
White Mountain NF, NH |
AVIRIS |
81 |
56 |
PLSR |
0.69 |
0.23 (SEC) |
0.23 (SECV) |
Ollinger et al. (2002) |
|
Lignin |
Juniper - Coastal rainforest |
West-central Oregon |
AVIRIS |
9 |
1 |
MLR |
0.75 |
17.90 (SEE) |
Matson et al. (1994) |
||
Lignin |
Slash Pine |
Gainesville, FL |
AVIRIS |
14 |
4 |
MLR |
0.98 |
Curran et al. (1997) |
|||
Lignin |
Chaparral |
Santa Monica, CA |
AVIRIS |
23 |
1 |
MLR |
0.81 |
5.39 (RMSE) |
Serrano et al. (2002) |
||
N |
N Hardwood and needleleaf |
Wisconsin |
AIS |
20 |
1 |
MLR |
0.83 |
0.04 (SE) |
Wessman et al. (1989) |
||
N |
Juniper - Coastal rainforest |
West-central Oregon |
AVIRIS |
9 |
1 |
MLR |
0.72 |
2.40 (SEE) |
Matson et al. (1994) |
||
N |
Juniper - Coastal rainforest |
West-central Oregon |
AVIRIS |
25 |
4 |
MLR |
0.90 |
0.70 (SEC) |
Johnson et al.(1994) |
||
N |
Slash Pine |
Gainesville, FL |
AVIRIS |
14 |
4 |
MLR |
0.98 |
Curran et al. (1997) |
|||
N |
N Hardwood and needleleaf |
Harvard Forest, MA |
AVIRIS |
21 |
1 |
MLR |
0.87 |
0.23 (SECV) |
0.83 |
0.27 (SEP) |
Martin and Aber (1997) |
N |
N Hardwood and needleleaf |
Blackhawk Island, WI |
AVIRIS |
20 |
1 |
MLR |
0.85 |
0.15 (SECV) |
0.75 |
1.32 (SEP) |
Martin and Aber (1997) |
N |
Chaparral |
Santa Monica, CA |
AVIRIS |
23 |
1 |
MLR |
0.75 |
0.55 (RMSE) |
Serrano et al. (2002) |
||
N |
N Hardwood and needleleaf |
White Mountain NF, NH |
AVIRIS |
53 |
36 |
PLSR |
0.82 |
0.23 (SECV) |
Smith et al. (2002) |
||
N |
Eucalyptus spp. |
Tumbarumba, Australia |
Hyperion |
14 |
1 |
PLSR |
0.95 |
0.11 (SEC) |
0.68 |
0.27 (SECV) |
Coops et al. (2003) |
N |
Eucalyptus spp. |
Tumbarumba, Australia |
Hyperion |
14 |
1 |
MLR |
0.83 |
0.10 (SEC) |
Coops et al. (2003) |
||
N |
N Hardwood and needleleaf |
Bartlett Exp. Forest, NH |
AVIRIS |
49 |
1 |
PLSR |
0.83 |
0.17 (SEC) |
0.79 |
0.19 (RMSEP) |
Smith et al. (2003) |
N |
N Hardwood and needleleaf |
Bartlett Exp. Forest, NH |
Hyperion |
49 |
1 |
PLSR |
0.82 |
0.17 (SEC) |
0.60 |
0.25 (RMSEP) |
Smith et al. (2003) |
N |
Deciduous Oak |
Green Ridge SF, MD |
Hyperion |
20 |
1 |
PLSR |
0.97 |
Townsend et al. (2003) |
|||
N |
Deciduous Oak |
Green Ridge SF, MD |
AVIRIS |
17 |
1 |
PLSR |
0.84 |
Townsend et al. (2003) |
|||
N |
N Hardwood and needleleaf |
Bartlett Exp. Forest, NH |
AVIRIS |
56 |
1 |
PLSR |
0.83 |
0.17(SEC) |
0.70 |
0.19(RMSEP) |
Ollinger and Smith (2005) |
N |
N Hardwood and needleleaf |
Bartlett Exp. Forest, NH |
Hyperion |
56 |
1 |
PLSR |
0.82 |
0.17(SEC) |
0.25 |
0.25(RMSEP) |
Ollinger and Smith (2005) |
N |
N Hardwood and boreal |
Adirondacks, NY |
Hyperion |
28 |
2 |
PLSR |
0.93 |
0.28(%) |
McNeil et al. (2008) |
||
N |
Mixed needleleaf-broadleaf |
Switzerland |
HyMap |
28 |
4 |
B&B |
0.53 |
0.38 (RMSE) |
Huber et al. (2008) |
||
N |
Various |
USA, Costa Rica, Australia |
AVIRIS |
42-75 |
5 |
PLSR |
0.83 |
0.14 (SEC) |
0.19 (SECV) |
Martin et al. (2008) |
|
N |
Various |
USA, Costa Rica, Australia |
Hyperion |
42-75 |
6 |
PLSR |
0.82 |
0.22 (SEC) |
0.25 (SECV) |
Martin et al. (2008) |
|
N |
Picea abies |
Gerolstein, Germany |
HyMap |
13 |
1 |
MLR |
0.57 |
0.05 (RMSE) |
Schlerf et al. (2010) |
||
N |
Needleleaf |
Vancouver Island, Canada |
AVIRIS |
17 |
1 |
PLSR |
0.77 |
0.21 (SECV) |
Hilker et al. (2012) |
||
N |
Needleleaf, Eucalyptus spp. |
Tumut, NSW, Australia |
HyMap |
80 |
5 |
PLSR |
0.54 |
0.90 (SEC) |
0.11 (SECV) |
Youngentob et al. (2012) |
|
N |
Needleleaf, Eucalyptus spp. |
Tumut, NSW, Australia |
HyMap |
80 |
5 |
MLR |
0.60 |
0.10 (SEC) |
0.10 (SECV) |
Youngentob et al. (2012) |
|
N |
Needleleaf, Eucalyptus spp. |
Tumut, NSW, Australia |
HyMap |
80 |
5 |
MLR |
0.58 |
0.10 (SEC) |
0.10 (SECV) |
Youngentob et al. (2012) |
|
N |
Sagebrush |
Eastern Idaho |
HyMap |
35 |
1 |
PLSR |
0.95 |
0.56 |
0.25 (PRESS) |
Mitchell et al. (2012) |
Table A2. Estimates of foliar traits measured in this study stratified by dominant canopy species. Species sorted by leaf habit (needleleaf, deciduous), and by relative frequency (%) in the overall dataset. Estimates of mean traits are presented along with standard deviations.
N% |
Marea |
C% |
ADF% |
ADL% |
Cellulose% |
δ15N‰ |
||||||||||
Sp. Code |
Species |
Rel. Freq (%) |
Mean |
S.D. |
Mean |
S.D. |
Mean |
S.D. |
Mean |
S.D. |
Mean |
S.D. |
Mean |
S.D. |
Mean |
S.D. |
Needleleaf |
||||||||||||||||
PIST |
Pinus stroba |
13.41 |
1.70 |
0.220 |
156.12 |
33.128 |
50.84 |
0.600 |
43.31 |
3.943 |
26.17 |
2.941 |
17.12 |
1.525 |
-1.11 |
1.172 |
PIRE |
Pinus resinosa |
7.66 |
1.43 |
0.211 |
190.70 |
15.563 |
50.87 |
0.581 |
46.50 |
2.129 |
26.68 |
1.316 |
18.81 |
1.343 |
-1.59 |
0.859 |
TSCA |
Tsuga canadensis |
4.21 |
1.75 |
0.272 |
116.14 |
16.517 |
49.95 |
0.308 |
30.23 |
4.271 |
15.95 |
2.572 |
13.79 |
1.910 |
-4.97 |
0.832 |
PIBA |
Pinus banksiana |
4.21 |
1.61 |
0.241 |
177.29 |
12.403 |
50.76 |
0.510 |
50.33 |
2.469 |
28.92 |
1.423 |
21.02 |
1.072 |
-4.48 |
0.350 |
ABBA |
Abies balsamifera |
2.68 |
1.64 |
0.148 |
149.74 |
23.107 |
51.79 |
1.113 |
40.02 |
3.409 |
23.42 |
2.140 |
14.87 |
1.568 |
-3.65 |
1.396 |
PIRU |
Pinus rubens |
1.92 |
1.40 |
0.262 |
150.87 |
6.423 |
50.71 |
0.742 |
44.28 |
5.162 |
24.68 |
1.637 |
17.80 |
4.043 |
-6.05 |
1.184 |
THOC |
Thuja occidentalis |
1.92 |
1.44 |
0.364 |
168.69 |
20.953 |
49.86 |
0.610 |
39.56 |
2.622 |
24.48 |
2.587 |
15.47 |
1.181 |
-3.91 |
1.277 |
PIMA |
Pinus mariana |
1.15 |
1.02 |
0.047 |
201.82 |
22.306 |
50.68 |
0.169 |
46.88 |
4.283 |
25.18 |
2.393 |
21.28 |
1.332 |
-6.03 |
0.215 |
JUVI |
Juniperous virginiana |
0.77 |
2.16 |
0.029 |
205.90 |
5.781 |
48.73 |
0.042 |
40.51 |
0.618 |
26.86 |
0.480 |
17.01 |
0.151 |
-1.91 |
0.146 |
LALA |
Larix larcinia |
0.77 |
1.50 |
0.103 |
168.59 |
7.197 |
50.16 |
0.031 |
50.17 |
0.650 |
30.58 |
0.075 |
19.50 |
0.713 |
-6.01 |
0.289 |
PISY |
Pinus sylvatica |
0.77 |
2.14 |
0.108 |
161.53 |
5.096 |
50.42 |
0.162 |
42.62 |
0.685 |
23.81 |
0.620 |
18.42 |
0.022 |
-1.86 |
0.056 |
PIAB |
Picea abies |
0.38 |
2.48 |
- |
134.14 |
- |
49.48 |
- |
35.53 |
- |
21.51 |
- |
14.98 |
- |
-3.00 |
- |
PIVI |
Pnius virginiana |
0.38 |
1.71 |
- |
136.64 |
- |
50.54 |
- |
39.34 |
- |
22.06 |
- |
18.31 |
- |
-3.43 |
- |
Broadleaf |
||||||||||||||||
ACSM |
Acer saccharum |
17.62 |
2.48 |
0.321 |
77.60 |
12.999 |
49.03 |
0.840 |
32.10 |
5.034 |
17.58 |
3.457 |
14.70 |
2.079 |
-3.72 |
1.141 |
QUAL |
Quercus alba |
6.90 |
2.86 |
0.212 |
92.66 |
10.772 |
49.44 |
0.478 |
33.40 |
1.951 |
19.29 |
1.319 |
15.33 |
0.521 |
-3.48 |
0.469 |
QURU |
Quercus rubrum |
6.51 |
2.75 |
0.184 |
97.14 |
9.028 |
49.78 |
0.835 |
36.47 |
3.005 |
22.51 |
2.541 |
15.70 |
1.393 |
-3.16 |
0.782 |
FAGR |
Fagus grandifolia |
4.21 |
2.20 |
0.332 |
82.02 |
12.634 |
50.21 |
0.567 |
43.01 |
2.940 |
24.93 |
1.973 |
17.91 |
1.929 |
-5.79 |
0.743 |
ACRU |
Acer rubrum |
3.45 |
2.28 |
0.264 |
82.25 |
12.101 |
49.81 |
0.877 |
34.84 |
5.373 |
19.72 |
3.178 |
15.40 |
2.698 |
-4.39 |
1.202 |
POTR |
Populus tremuloides |
3.07 |
2.56 |
0.243 |
79.84 |
13.786 |
50.35 |
0.804 |
35.60 |
3.292 |
25.51 |
3.653 |
17.62 |
1.491 |
-2.45 |
0.534 |
ACSN |
Acer saccharinium |
2.68 |
2.52 |
0.110 |
85.61 |
4.615 |
49.68 |
0.648 |
24.71 |
4.436 |
15.07 |
2.182 |
10.83 |
2.063 |
-2.17 |
0.838 |
QUPR |
Quercus prinoides |
1.92 |
2.30 |
0.321 |
92.94 |
16.650 |
49.70 |
0.578 |
36.74 |
2.525 |
21.74 |
1.882 |
16.09 |
0.935 |
-2.99 |
0.832 |
LITU |
Liriodendron tulipifera |
1.53 |
2.60 |
0.132 |
91.43 |
15.222 |
48.69 |
0.648 |
32.95 |
1.183 |
19.55 |
0.825 |
13.77 |
0.903 |
-1.88 |
0.766 |
PODE |
Populus deltoides |
1.53 |
2.64 |
0.147 |
82.53 |
1.982 |
48.98 |
0.251 |
30.81 |
1.718 |
19.42 |
0.920 |
12.20 |
0.838 |
-1.05 |
0.399 |
CAOV |
Carya ovata |
1.15 |
2.86 |
0.328 |
84.70 |
29.652 |
47.65 |
0.881 |
36.88 |
0.506 |
20.10 |
2.372 |
16.99 |
1.327 |
-2.80 |
0.975 |
FRNI |
Fraxinus nigra |
1.15 |
2.80 |
0.050 |
85.87 |
7.002 |
47.85 |
0.345 |
28.21 |
4.989 |
16.93 |
1.729 |
11.71 |
2.805 |
-1.29 |
0.807 |
QUMA |
Quercues macrocarpa |
1.15 |
2.82 |
0.198 |
103.91 |
7.179 |
48.91 |
0.465 |
35.61 |
0.766 |
20.87 |
0.793 |
16.09 |
0.144 |
-3.16 |
0.729 |
ROPS |
Robinia pseudoacacia |
1.15 |
3.21 |
0.167 |
64.32 |
6.954 |
48.26 |
0.651 |
35.86 |
1.992 |
23.15 |
1.306 |
16.81 |
0.392 |
-1.11 |
0.439 |
TIAM |
Tilia americana |
1.15 |
2.82 |
0.194 |
88.82 |
2.871 |
48.49 |
0.762 |
35.18 |
1.665 |
21.36 |
0.832 |
15.70 |
0.827 |
-2.50 |
0.417 |
CEOC |
Celtis occidentalis |
0.77 |
2.98 |
0.033 |
66.71 |
2.060 |
46.59 |
0.286 |
28.84 |
1.282 |
17.46 |
0.423 |
13.78 |
0.816 |
-0.74 |
0.208 |
FRPE |
Fraxinus pennsylvanica |
0.77 |
2.68 |
0.269 |
70.37 |
13.070 |
47.23 |
0.278 |
31.64 |
0.688 |
18.56 |
0.075 |
14.82 |
0.940 |
-2.99 |
1.068 |
NYSY |
Nyssa sylvatica |
0.77 |
2.40 |
0.065 |
81.28 |
0.948 |
49.62 |
0.358 |
32.22 |
0.217 |
18.00 |
0.034 |
14.22 |
0.224 |
-3.67 |
0.032 |
QUEL |
Quercues ellipsoides |
0.77 |
2.96 |
0.187 |
94.99 |
14.752 |
49.84 |
0.253 |
33.95 |
0.157 |
20.52 |
1.474 |
15.25 |
0.259 |
-3.19 |
0.918 |
CASP |
Carya spp. |
0.38 |
2.27 |
- |
129.13 |
- |
49.47 |
- |
37.12 |
- |
23.16 |
- |
15.26 |
- |
-1.86 |
- |
JUNI |
Juglans nigra |
0.38 |
2.55 |
- |
82.34 |
- |
47.96 |
- |
18.02 |
- |
8.27 |
- |
9.75 |
- |
-1.72 |
- |
PRSE |
Prunus serotina |
0.38 |
3.04 |
- |
99.55 |
- |
47.98 |
- |
32.70 |
- |
20.44 |
- |
13.86 |
- |
-1.53 |
- |
RHCA |
Rhamnus cathartica |
0.38 |
3.22 |
- |
84.95 |
- |
48.11 |
- |
33.43 |
- |
21.59 |
- |
14.20 |
- |
-1.19 |
- |
Table A3. Leaf to canopy trait aggregation schemes used in model building. Note that results from aggregation scheme A (Top of canopy) are reported in detail in the manuscript.
|
|
|
Canopy weighting |
||
Scheme |
Weight |
|
Top |
Mid |
Bottom |
A |
Top of canopy |
|
90% |
9% |
1% |
B |
Mid-weighted canopy |
|
64.5% |
32.3% |
3.2% |
C |
Whole canopy |
|
40% |
40% |
20% |
D |
Only top canopy |
|
100% |
0% |
0% |
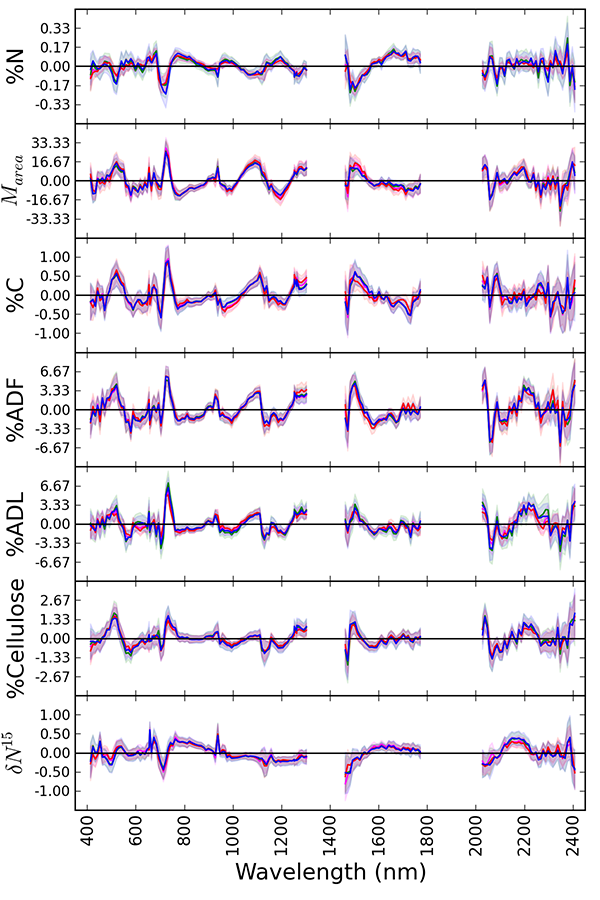
Fig. A1. Comparisons of PLSR coefficients obtained by fitting models to foliar traits aggregated according to different schemes described in Table A3; A (green), B (magenta), C (red), D (blue). Overlapping lines (and error estimates) indicate close agreement between all models regardless of aggregation scheme.
Fig. A2. Model fits obtained by using aggregations scheme A.
Fig. A3. Model fits obtained by using aggregations scheme B.
Fig. A4. Model fits obtained by using aggregations scheme C.
Fig. A5. Model fits obtained by using aggregations scheme D.
Fig. A6. False color composites of foliar traits (left panels; R/G/B = %ADL/Marea/%N) compared with NLCD 2006 landcover maps (right panels). Foliar trait association maps provide richer information on foliar traits across forest ecotones than discrete classes. Subplot locations are 1: Ottawa NF, MI, 2: Green Ridge SF, MD; 3: Porcupine Mountains SF, MI (boxes B, F and A in Fig. 1, Landcover classification legend is Figure A9).
Fig. A7. Foliar trait association maps (subplot 1: false color composite R/G/B = %ADL/ Marea/%Nitrogen) provide richer detail than NLCD 2006 landcover classifications (subplot 6), or from fall aerial imagery (subplot 2; 11/08/2010 GoogleEarth™). While leaf-off aerial imagery (subplot D; 4/14/2005 GoogleEarth™) clearly identifies needleleaf forest stands (also see high lignin+Marea [yellow] areas in subplot 1), color enhancement of fall aerial imagery (subplot 3) shows phenological differences (subplot 4) between dominant deciduous species (Quercus rubrum, Acer saccharum) corresponding to spatial patterns of foliar traits in subplot A. Subplot 5 indicates high confidence in mapping traits (%N, S.D. shown) across deciduous forest landcover. High prediction uncertainties are only observed in edges or non-forest areas.
Fig. A8. Recently disturbed regions show up as sharp boundaries in trait association maps (subplot 1: false color composite R/G/B = %ADL/Marea/%Nitrogen, compare with subplot 2: landcover from NLCD 2006). Changes in foliar nutrient content (%N subplot 3) and elevated uncertainties (subplot 4) capture logging activities (pre-cut subplot 5: 8/24/2007, post-logging subplot 6 10/19/2009, images from GoogleEarth™) near the Fernow Experimental Forest, WV.
Fig. A9. Legend from the National Land Cover Database 2006 (www.mrlc.gov/nlcd06_leg.php).
Literature cited
Coops, N. C., M. L. Smith, M. E. Martin, and S. V. Ollinger. 2003. Prediction of eucalypt foliage nitrogen content from satellite-derived hyperspectral data. Ieee Transactions on Geoscience and Remote Sensing 41:1338-1346.
Curran, P. J., J. A. Kupiec, and G. M. Smith. 1997. Remote sensing the biochemical composition of a slash pine canopy. Ieee Transactions on Geoscience and Remote Sensing 35:415-420.
Hilker, T., L. Lepine, N. C. Coops, R. S. Jassal, T. A. Black, M. A. Wulder, S. Ollinger, O. Tsui, and M. Day. 2012. Assessing the impact of N-fertilization on biochemical composition and biomass of a Douglas-fir canopy-A remote sensing approach. Agricultural and Forest Meteorology 153:124-133.
Huber, S., M. Kneubuhler, A. Psomas, K. Itten, and N. E. Zimmermann. 2008. Estimating foliar biochemistry from hyperspectral data in mixed forest canopy. Forest Ecology and Management 256:491-501.
Johnson, L. F., C. A. Hlavka, and D. L. Peterson. 1994. Multivariate-analysis of aviris data for canopy biochemical estimation along the oregon transect. Remote Sensing of Environment 47:216-230.
Martin, M. E., and J. D. Aber. 1997. High spectral resolution remote sensing of forest canopy lignin, nitrogen, and ecosystem processes. Ecological Applications 7:431-443.
Martin, M. E., L. C. Plourde, S. V. Ollinger, M. L. Smith, and B. E. McNeil. 2008. A generalizable method for remote sensing of canopy nitrogen across a wide range of forest ecosystems. Remote Sensing of Environment 112:3511-3519.
Matson, P., L. Johnson, C. Billow, J. Miller, and R. L. Pu. 1994. Seasonal patterns and remote spectral estimation of canopy chemistry across the Oregon transect. Ecological Applications 4:280-298.
McNeil, B. E., J. M. Read, T. J. Sullivan, T. C. McDonnell, I. J. Fernandez, and C. T. Driscoll. 2008. The spatial pattern of nitrogen cycling in the Adirondack Park, New York. Ecological Applications 18:438-452.
Mitchell, J. J., N. F. Glenn, T. T. Sankey, D. R. Derryberry, and M. J. Germino. 2012. Remote sensing of sagebrush canopy nitrogen. Remote Sensing of Environment 124:217-223.
Ollinger, S. V., and M. L. Smith. 2005. Net primary production and canopy nitrogen in a temperate forest landscape: An analysis using imaging spectroscopy, modeling and field data. Ecosystems 8:760-778.
Ollinger, S. V., M. L. Smith, M. E. Martin, R. A. Hallett, C. L. Goodale, and J. D. Aber. 2002. Regional variation in foliar chemistry and N cycling among forests of diverse history and composition. Ecology 83:339-355.
Schlerf, M., C. Atzberger, J. Hill, H. Buddenbaum, W. Werner, and G. Schuler. 2010. Retrieval of chlorophyll and nitrogen in Norway spruce (Picea abies L. Karst.) using imaging spectroscopy. International Journal of Applied Earth Observation and Geoinformation 12:17-26.
Serrano, L., J. Penuelas, and S. L. Ustin. 2002. Remote sensing of nitrogen and lignin in Mediterranean vegetation from AVIRIS data: Decomposing biochemical from structural signals. Remote Sensing of Environment 81:355-364.
Smith, M. L., M. E. Martin, L. Plourde, and S. V. Ollinger. 2003. Analysis of hyperspectral data for estimation of temperate forest canopy nitrogen concentration: Comparison between an airborne (AVIRIS) and a spaceborne (Hyperion) sensor. Ieee Transactions on Geoscience and Remote Sensing 41:1332-1337.
Smith, M. L., S. V. Ollinger, M. E. Martin, J. D. Aber, R. A. Hallett, and C. L. Goodale. 2002. Direct estimation of aboveground forest productivity through hyperspectral remote sensing of canopy nitrogen. Ecological Applications 12:1286-1302.
Townsend, P. A., J. R. Foster, R. A. Chastain, and W. S. Currie. 2003. Application of imaging spectroscopy to mapping canopy nitrogen in the forests of the central Appalachian Mountains using Hyperion and AVIRIS. Ieee Transactions on Geoscience and Remote Sensing 41:1347-1354.
Wessman, C. A., J. D. Aber, and D. L. Peterson. 1989. An evaluation of imaging spectrometry for estimating forest canopy chemistry. International Journal of Remote Sensing 10:1293-1316.
Youngentob, K. N., L. J. Renzullo, A. A. Held, X. P. Jia, D. B. Lindenmayer, and W. J. Foley. 2012. Using imaging spectroscopy to estimate integrated measures of foliage nutritional quality. Methods in Ecology and Evolution 3:416-426.