Ecological Archives A025-122-A3
Erik R. Schoen, David A. Beauchamp, Anna R. Buettner, and Nathanael C. Overman. 2015. Temperature and depth mediate resource competition and apparent competition between Mysis diluviana and kokanee. Ecological Applications 25:1962–1975. http://dx.doi.org/10.1890/14-1822.1
Appendix C. Bioenergetics models for Mysis and kokanee: detailed methods and consumption : production ratios.
Inputs to bioenergetics models for Mysis and kokanee
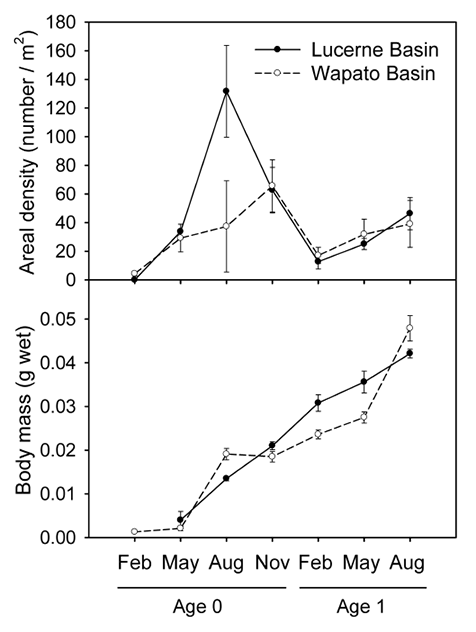
We quantified growth of Mysis by tracking the mean wet mass of each age class (Fig. C1). We measured Mysis individuals for body length, from the tip of the rostrum to the tip of the telson, and for blotted wet mass. We identified Mysis age classes from modes in length-frequency histograms. Mysis exhibited a 1.5-year life span, with juveniles released by females between February and May and the parental generation senescing by the following November. Total Mysis growth from juvenile to adult was similar among lake basins, but seasonal growth patterns differed slightly (Fig. C1). Mysis in Wapato Basin grew rapidly between May and August but grew more slowly during the rest of the year, while Mysis grew at a relatively consistent rate year round in Lucerne Basin. To determine whether size-selective predation negatively biased estimates of Mysis growth in the Wapato Basin during August, when growth appeared to be slow or slightly negative, we compared the length distribution of mysids collected in net tows to the length distribution of Mysis found in lake trout and burbot Lota lota stomachs collected during that period. We reconstructed the body lengths of partially digested Mysis based on lengths of eye stalks, which were usually intact in stomach samples (Gal et al. 2006). We derived a linear regression relating body length (BL) to eye-stalk length (EL), both measured in mm, using intact mysids collected by net (n = 90, r² = 0.87, p < 0.001):
BL = 144.7 EL – 1.70. (C.1)
Mysis in stomachs were slightly larger than Mysis collected in nets, suggesting that predation was weakly size selective, although this difference was not significant (t = 1.471, df = 225, P = 0.14).
We characterized kokanee growth with a mass-at-age relationship. We calculated mean wet mass for age 1, 2, and 3 kokanee captured during May and for maturing kokanee captured in August, which were preparing to spawn at age 4 (Table C2). Kokanee grew to a mean body mass of 262.1 ± 8.6 g at age 3 in August (mean ± SE). We estimated the mass of age-0 kokanee in May from the mean acoustic target strength of age-0 kokanee detected during the May survey, using Love’s (1971) target strength-total length relationship, a total length to fork length relationship for kokanee (Hyatt and Hubert 2000), and a length-mass relationship for Lake Chelan kokanee (Schoen et al. 2012). We treated kokanee as a single population moving between Wapato and Lucerne Basins, with identical growth inputs in each basin.
We estimated the thermal experience of Mysis as the mean temperature of the hypolimnion, metalimnion, and the depth reached at the apex of the vertical migration, weighted by time spent in each depth zone (Table C3). We assumed mysids spent the daylight and civil twilight period in the hypolimnion, one hour in the metalimnion during each of the upward and downward migrations, and the remainder of the diel period at the apex depth (seasonal daylight data for Chelan, WA from US Naval Observatory, Astronomical Applications Department: <http://aa.usno.navy.mil>). We estimated the thermal experience of kokanee as the mean temperature of the metalimnion or the mean temperature of the upper 50 m of the water column during non-stratified periods (Table C4).
We estimated the diet compositions of Mysis and kokanee from field and literature data. We estimated the proportions of phytoplankton and zooplankton in the diets of juvenile Mysis (length < 8 mm) and adult Mysis with a simple two-source linear mixing model (Phillips and Gregg 2001) using stable isotope signatures from Lake Chelan (Schoen and Beauchamp 2010). We used filter-feeding clams (Corbicula spp.) as a surrogate for zooplankton to reduce isotopic variability, and we assumed that clams and zooplankton were herbivorous, with a trophic fractionation rate of 3.4‰ δ15N (Post 2002). This model assigned juvenile Mysis a fully herbivorous diet, in agreement with literature data (Rybock 1978, Chipps 1997, Johannsson et al. 2001).; The model assigned adult Mysis a diet of 70% zooplankton and 30% phytoplankton that was similar to the omnivorous diets reported in other oligotrophic lakes (Grossnickle 1982, Johannsson et al. 2001, Nordin et al. 2007). We determined the basin-specific, seasonal diet composition of kokanee from stomach samples collected between May and August (our table C4; Brown 1984). Kokanee diets collected in Wapato Basin during May and June consisted predominantly of Diacyclops (age 1 kokanee) or chironomids, Daphnia, and Bosmina (ages 2 and 3 kokanee). In the Lucerne Basin during August, kokanee ate Daphnia and Bosmina almost exclusively. When field data were not available, we assumed the proportions of cladocerans and copepods in the kokanee diet were equal to their relative abundance in net samples, except we assumed kokanee consumed exclusively cladocerans when the cladoceran density exceeded 0.4 animals / L, a prey-switching threshold for juvenile sockeye salmon (O. nerka) (Scheuerell et al. 2005).
We compiled energy densities of Mysis,kokanee, and their prey from literature values (Tables C3 and C4). We used reported energy densities of adult and juvenile Mysis (Lasenby 1971) for May and August, and the adult value was reduced by 20% during November and February to account for diminished Mysis lipid reserves during winter (Adare and Lasenby 1994, Chipps and Bennett 2000). The energy density of kokanee varied with body size following Beauchamp et al. (1989). We adjusted the energy density of zooplankton prey seasonally for Mysis. We used the energy density of cladocerans (1620 J / g) during August, when Daphnia and Bosmina were abundant, and we used the energy density of copepods (2260 J / g; Luecke and Brandt 1993) during all other months. We used a higher energy density of 3800 J / g for cladocerans consumed by kokanee to account for water squeezed out of the carapace during ingestion, and we corrected the model outputs to indicate the biomass of live cladocerans removed from the lake (Luecke and Brandt 1993, Stockwell et al. 1999).
Literature Cited
Adare, K. I., and D. C. Lasenby. 1994. Seasonal changes in the total lipid content of the opossum shrimp, Mysis relicta (Malacostraca, Mysidacea). Canadian Journal of Fisheries and Aquatic Sciences 51:1935–1941.
Beauchamp, D. A., D. J. Stewart, and G. L. Thomas. 1989. Corroboration of a bioenergetics model for sockeye salmon. Transactions of the American Fisheries Society 118:597–607.
Brown, L. G. 1984. Lake Chelan fishery investigations. Chelan County Public Utility District No. 1 and Washington Department of Game, Olympia, Washington, USA.
Chipps, S. R. 1997. Mysis relicta in Lake Pend Oreille: seasonal energy requirements and implications for mysid-cladoceran interactions. Doctoral dissertation. University of Idaho.
Chipps, S. R. and D. H. Bennett. 2000. Zooplanktivory and nutrient regeneration by invertebrate (Mysis relicta) and vertebrate (Oncorhynchus nerka) planktivores: Implications for trophic interactions in oligotrophic lakes. Transactions of the American Fisheries Society 129:569–583.
Cummins, K. W. and J. C. Wuycheck. 1971. Caloric equivalents for investigations in ecological energetics. Mitteilungen Internationale Vereinigung für Theoretische und Angewandte Limnologie 18:158 p.
Gal, G., L. G. Rudstam, E. L. Mills, J. R. Lantry, O. E. Johannsson, and C. H. Greene. 2006. Mysid and fish zooplanktivory in Lake Ontario: quantification of direct and indirect effects. Canadian Journal of Fisheries and Aquatic Sciences 63:2734–2747.
Grossnickle, N. E. 1982. Feeding habits of Mysis relicta: an overview. Hydrobiologia 93:101–107.
Hyatt, M. H. and W. A. Hubert. 2000. Proposed standard-weight (Ws) equations for kokanee, golden trout and bull trout. Journal of Freshwater Ecology 15:559–563.
Johannsson, O. E., M. F. Leggett, L. G. Rudstam, M. R. Servos, M. A. Mohammadian, G. Gal, R. M. Dermott, and R. H. Hesslein. 2001. Diet of Mysis relicta in Lake Ontario as revealed by stable isotope and gut content analysis. Canadian Journal of Fisheries and Aquatic Sciences 58:1975–1986.
Lasenby, D. C. 1971. The ecology of Mysis relicta in an arctic and a temperate lake. Doctoral dissertation. University of Toronto, Toronto.
Love, R. 1971. Dorsal aspect target strength of an individual fish. The Journal of the Acoustical Society of America 49:816.
Luecke, C. and D. Brandt. 1993. Estimating the energy density of daphnid prey for use with rainbow trout bioenergetics models. Transactions of the American Fisheries Society 122:386–389.
Nordin, L. J., M. T. Arts, O. E. Johannsson, and W. D. Taylor. 2007. An evaluation of the diet of Mysis relicta using gut contents and fatty acid profiles in lakes with and without the invader Bythotrephes longimanus (Onychopoda, Cercopagidae). Aquatic Ecology 42:421–436.
Phillips, D. L. and J. W. Gregg. 2001. Uncertainty in source partitioning using stable isotopes. Oecologia 127:171–179.
Post, D. M. 2002. Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecology 83:703–718.
Rybock, J. T. 1978. Mysis relicta Loven in Lake Tahoe: Vertical distribution and nocturnal predation. Doctoral dissertation. University of California-Davis.
Scheuerell, J. M., D. E. Schindler, M. D. Scheuerell, K. L. Fresh, T. H. Sibley, A. H. Litt, and J. H. Shepherd. 2005. Temporal dynamics in foraging behavior of a pelagic predator. Canadian Journal of Fisheries and Aquatic Sciences 62:2494–2501.
Schoen, E. R. and D. A. Beauchamp. 2010. Predation impacts of lake trout and Chinook salmon in Lake Chelan, Washington: Implications for prey species and fisheries management. Washington Cooperative Fish and Wildlife Research Unit, US Geological Survey, University of Washington, Seattle, Washington, USA.
Schoen, E. R., D. A. Beauchamp, and N. C. Overman. 2012. Quantifying latent impacts of an introduced piscivore: Pulsed predatory inertia of lake trout and decline of kokanee. Transactions of the American Fisheries Society 141:1191–1206.
Stockwell, J. D., K. L. Bonfantine, and B. M. Johnson. 1999. Kokanee foraging: A Daphnia in the stomach is worth two in the lake. Transactions of the American Fisheries Society 128:169–174.
Table C1. Ratios of the combined zooplankton consumption rate of the Mysis diluviana and kokanee populations to the production rates of cladocerans only, or cladocerans and copepods (C:P ratios) in Lucerne and Wapato Basins.
|
Cladocerans only |
|
Cladocerans + Copepods |
||
Month |
Lucerne |
Wapato |
Lucerne |
Wapato |
|
February |
12.04 |
0.91 |
0.56 |
0.56 |
0.24 |
May/June |
11.29 |
0.72 |
0.25 |
0.25 |
0.13 |
August |
0.28 |
0.08 |
0.08 |
0.08 |
0.04 |
November |
2.72 |
0.75 |
0.16 |
0.16 |
0.12 |
Table C2. Growth inputs used in bioenergetics simulations for kokanee.
Age |
Month |
Body mass (g wet) |
0 |
May |
1.25 |
1 |
May |
37.5 |
2 |
May |
165.8 |
3 |
May |
225.2 |
3 |
August |
262.1 |
Note: Age 3 kokanee captured during August were maturing adults preparing to spawn 1-2 months later at age 4.
Table C3. Thermal experience, diet composition (by mass), and energy density inputs used in bioenergetics simulations for Mysis diluviana.
Age |
Month |
|
Thermal experience (ºC) |
Mysis energy density (J / g wet) |
Diet composition |
||
Algae |
Zooplankton |
||||||
Lucerne |
Wapato |
(2558)† |
(1,620–2,260)‡ |
||||
0 |
May |
|
7.0 |
5.9 |
3135 |
1 |
0 |
0 |
August |
|
8.4 |
6.8 |
3720 |
0.3 |
0.7 |
0 |
November |
|
8.8 |
9.4 |
2976 |
0.3 |
0.7 |
1 |
February |
|
6.3 |
5.4 |
2976 |
0.3 |
0.7 |
1 |
May |
|
7.0 |
5.9 |
3720 |
0.3 |
0.7 |
1 |
August |
|
8.4 |
6.8 |
3720 |
0.3 |
0.7 |
Notes: Thermal experience is reported separately for Lucerne and Wapato Basins. The energy density (J / g wet mass) of each prey type is indicated in parentheses.
†Cummins and Wuycheck (1971).
‡The energy density of zooplankton varied seasonally (see text).
Table C4. Thermal experience, diet composition (by mass), and prey energy density inputs used in bioenergetics simulations for kokanee in Lucerne and Wapato Basins.
Month |
Thermal experience (ºC) |
Diet composition |
|||||||
Lucerne |
|
Wapato |
|||||||
Chironomids |
Cladocerans |
Copepods |
Chironomids |
Cladocerans |
Copepods |
||||
Lucerne |
Wapato |
(3400) |
(3800) |
(2260) |
|
(3400) |
(3800) |
(2260) |
|
Feb |
6.3 |
5.4 |
0.00 |
0.00 |
1.00 |
|
0.00 |
1.00 |
0.00 |
May |
8.0 |
9.4 |
0.00 |
0.00 |
1.00 |
|
0.49 |
0.48 |
0.03 |
Aug |
12.1 |
13.8 |
0.00 |
1.00 |
0.00 |
|
0.00 |
1.00 |
0.00 |
Nov |
9.1 |
8.5 |
0.00 |
0.01 |
0.99 |
|
0.00 |
1.00 |
0.00 |
Note: The energy density (J / g wet mass) of each prey type is indicated in parentheses.
Fig. C1. Density of Mysis diluviana in the upper 80 m of the water column at night (top panel). Mysis densities varied seasonally due to mortality and changes in the proportion of the population that ascended into the water column. Growth of Mysis (bottom panel). Mysis exhibited a 1.5 year life cycle with two overlapping generations present during February through August. Symbols represent means ± 1 standard error.