Ecological Archives A025-060-A1
J. R. Huver, J. Koprivnikar, P. T. J. Johnson, and S. Whyard. 2015. Development and application of an eDNA method to detect and quantify a pathogenic parasite in aquatic ecosystems. Ecological Applications 25:991–1002. http://dx.doi.org/10.1890/14-1530.1
Appendix A. Hand-held filter unit details.
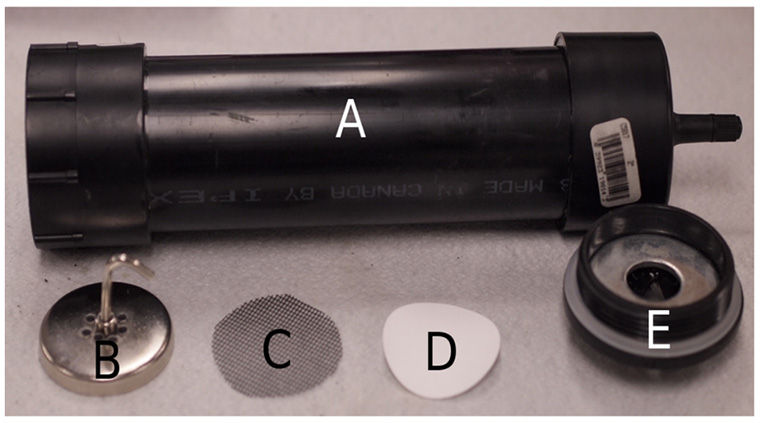
Our handheld filtration unit for the collection of water-borne eDNA in field samples represents a variation on methods used in previous studies, such as the use of small-pore membrane filters (e.g., Barnes et al. 2014) to gather particulate matter. Passive water flow, siphoning action, and persistaltic pumps can be employed (e.g. Worrell et al. 201, Goldberg et al. 2011) but we choose to create a hand-powered pumping unit in order to minimize collection time and equipment necessary in the field. Notably, while some researchers have opted to collect field water samples and filter these in the laboratory (e.g., Jerde et al. 2011), ease of multiple sampling at difficult to access sites was an important consideration for our methodology. The unit consisted of two main sections – the filter body and the filter support (see Fig. A1). The body was made of the following materials: 24.7 cm of acrylonitrile butadiene styrene (ABS) tubing (2" inside diameter), a 2" ABS threaded end cap adapter (female), a snap-in tubeless tire valve compatible with a standard bicycle pump (with pressure gauge), and a 2" ABS cap. We marked the inside of the tube to correspond to a volume of 500 mL. The support unit consisted of the following: a 2" ABS threaded end cap, a ¾" plate washer (2" outside diameter), a 2" round magnet (¾" thickness) drilled with 9 holes measuring 1/8" each, and fiberglass window screen mesh cut into a 2" diameter circle.
We cut a V-shaped grove across the threaded end cap of the support unit to allow water drainage from this end of the filtration apparatus and then glued the plate washer to the inside of the cap using a water-resistant epoxy. This washer ensured that the magnet would hold the cellulose nitrate filter in place during pumping. We used 3µm pore size Whatman cellulose nitrate membrane filters (Sigma-Aldrich) to collect particular matter and the fiberglass mesh acted to support the filter and prevent tearing. This was also minimized by not exceeding pressures of 40 PSI during pumping. The non-threaded ABS cap was attached to one end of the filter body with ABS solvent cement and we then drilled a hole in it large enough to insert the tire valve, sealing this with epoxy. We glued the female ABS threaded cap adapter onto the other end of the body (the open end) using ABS solvent cement as well.
In the field, we placed the mesh screen on the washer in the support unit and then put the membrane filter on top, followed by the magnet to ensure a firm seal. We lowered the filter body below the water level until the tube had filled to the 500 mL mark inside. The support piece was then screwed onto the body and the entire unit inverted to allow attachment of the pump and water drainage. After the water had been cleared from the filtration apparatus through pumping action, the support unit was unscrewed to allow removal of the membrane filter, which was immediately placed into a container with 70% ethanol. This procedure was performed 5 times at each field site (i.e., there were 5 subsamples/site) with a different membrane filter. A new filter apparatus was used at each separate site to prevent cross-contamination.
Fig. A1. Components of the handheld filtration unit for the collection of water-borne eDNA samples. The apparatus consisted of the filter body (A) and the filter support unit, with the latter made up of a magnet (B), mesh screen (C), cellulose nitrate filter (D), and cap (E). Photo by J.R. Huver.
Literature cited
Barnes, M. A., C. R. Turner, C. L. Jerde, M. A. Renshaw, W. L. Chadderton, and D. M. Lodge. 2014. Environmental conditions influence eDNA persistence in aquatic systems. Environmental Science and Technology 48:1819−1827.
Goldberg, C. S., D. S. Pilliod, R. S. Arkle, and L. P. Waits. 2011. Molecular detection of vertebrates in stream water: a demonstration using rocky mountain tailed frogs and Idaho giant salamanders. PLoS ONE 6 e22746.
Worrell, C., N. Xiao, J. E. Vidal, L. Chen, B. Zhong, and J. Remais. 2011. Field detection of Schistosoma japonicum cercariae in environmental water samples by quantitative PCR. Applied and Environmental Microbiology 77:2192–2195.