Appendix D. Crop flowering parameter values.
The crop-specific parameter values for our model are summarized in Table A1;
The basis for these values is described as follows. In all cases, ![]() and
and ![]() were estimated by fitting the pollen concentration or number of open flowers to a normal distribution by minimising the error sums of squares, using the “Solver” routine from Excel (Microsoft Corp.).
were estimated by fitting the pollen concentration or number of open flowers to a normal distribution by minimising the error sums of squares, using the “Solver” routine from Excel (Microsoft Corp.).
Sugar beet (Beta vulgaris)
Sugar beet is one of the most important crops of the cool temperate regions (Eastham and Sweet 2002). It is harvested for the roots it produces at the end of the first growing season. The root crop is purely vegetative, and hence pollination has no influence on the composition of the roots and foliage (Ingram 2000). However, a few plants (“bolters”) per hectare do flower (Treu and Emberlin 2000). The seed produced by bolters could result in GM annual weed beet occurring in the next root crop. There is then the risk of cross-pollination if the weed beet is harvested and processed with the surrounding non-GM crop (Eastham and Sweet 2002).
There is a medium to high risk of crop-to-crop gene flow in sugar beet in the European Union (Eastham and Sweet 2002).
Sugar beet is mostly wind pollinated, although insect pollination also occurs (Eastham and Sweet 2002). Beet pollen may travel over considerable distances (Eastham and Sweet 2002).
(a) Pollen release: diurnal variation (![]() )
)
There is little literature concerning the release of pollen from sugar beet. Scott (1970) describes the release of pollen from fields of diploid seed crop sugar beet over three years (1965–1967), in each year from mid-June to mid-August, in Suffolk, UK. The density of pollen in the air averaged over a single day varied little among years. Therefore we estimated s diurnal from a single year, 1967.
The time of maximum pollen concentration (hmax) was approximately 11:00, and ![]() = 2.8 hours (Fig. D1).
= 2.8 hours (Fig. D1).
(b) Pollen release: seasonal variation (![]() )
)
We found that ![]() = 7.9 days from the average five-day running mean concentration of pollen in the air over the three years from Scott (1970) (Fig. D2). In addition, Scott (1967) found that sugar beet flowers over at least five weeks, which is consistent with our estimate of the
= 7.9 days from the average five-day running mean concentration of pollen in the air over the three years from Scott (1970) (Fig. D2). In addition, Scott (1967) found that sugar beet flowers over at least five weeks, which is consistent with our estimate of the ![]() .
.
Peak flowering of sugar beet is in the range end June to July in the UK (A. Guthrie, personal communication). Peak flowering (tmax) was set at 1st July.
(c) Stigma reception: diurnal variation
Beet flowers open in the morning, and the anthers dehisce before noon ( McGregor 1976 ). The stigmatic lobes open gradually in the afternoon and are not fully open until the second or even the third day ( McGregor 1976 ). We assume that there is no diurnal variation in stigma reception during the daytime.
(d) Stigma reception: seasonal variation
Beet stigmas may be receptive for more than 2 weeks ( McGregor 1976 ).
Oilseed rape (Brassica napus)
There is a high risk of crop-to-crop gene flow in oilseed rape in the European Union (Eastham and Sweet 2002). Oilseed rape is only partially wind-pollinated. In arable fields of winter-sown oilseed rape, pollination occurs primarily by autonomous mechanisms, with some contribution from the wind and insects (Hayter and Cresswell 2006).
(a) Pollen release: diurnal variation (![]() )
)
McCartney and Lacey (1991) measured the diurnal variation in pollen air concentration above fields of oilseed rape for three days within each of five years (1985–1989). The average of these 15 readings is given in Fig. D3(a) (taken from McCartney and Lacey (1991), Fig. 2). Pollen concentration peaked at about 14:00 hours. There was practically no pollen in the air between 23:00 and 7:00 (Fig. D3a). The best fit to a normal distribution gave ![]() = 3.3 hours.
= 3.3 hours.
Williams (1984) recorded the pollen air concentration at the edge of a field of oilseed rape at Rothamsted, UK over 38 days. We read off the relevant data from Fig. 3 (Williams 1984). There was very little airborne pollen between 21:00 and 8:00 hours, in agreement with McCartney and Lacey (1991) (Fig. D3b). However, contrary to McCartney and Lacey (1991), Williams (1984) found two peaks: at 11:30 and at 14:30. Fitting one normal distribution to the data gave a mean at 13:00 and ![]() = 3.2 hours, very close to the McCartney and Lacey (1991) value.
= 3.2 hours, very close to the McCartney and Lacey (1991) value.
The average of the values from the two studies gives ![]() = 3.2 hours, with peak pollen release (hmax) at approximately 14:00.
= 3.2 hours, with peak pollen release (hmax) at approximately 14:00.
(b) Pollen release: seasonal variation (![]() )
)
McCartney and Lacey (1991) also recorded the seasonal variation in pollen air concentration in each of five years. The mean of these five series was fitted to a normal distribution (Fig. D4a). In this case, ![]() = 7.8 days.
= 7.8 days.
Scheffler et al. (1995) measured the proportion of oilseed rape plants flowering in each of 4 plots over 7 days during the peak of the flowering period in 1992. We assumed that the relative pollen concentration is proportional to the proportion of plants flowering. The data was read from Fig. 2 of Scheffler et al. (1995). The average proportion of plants flowering over the 4 plots for each of the 7 days was fitted to a normal distribution (Fig. D4b). In this case ![]() = 7.9 days, very close to the figure from McCartney and Lacey (1991).
= 7.9 days, very close to the figure from McCartney and Lacey (1991).
The average of the values from the two studies gives ![]() = 7.8 days. Winter oilseed rape flowers in April to early May in northern continental Europe, and in June and July in Scotland and Scandinavia (Eastham and Sweet 2002). The majority of spring oilseed rape flowers at least one month later (Eastham and Sweet 2002). Peak flowering of winter and spring oilseed rape (tmax) was set at 15th April and 15th June, respectively.
= 7.8 days. Winter oilseed rape flowers in April to early May in northern continental Europe, and in June and July in Scotland and Scandinavia (Eastham and Sweet 2002). The majority of spring oilseed rape flowers at least one month later (Eastham and Sweet 2002). Peak flowering of winter and spring oilseed rape (tmax) was set at 15th April and 15th June, respectively.
(c) Stigma reception: diurnal variation
The stigma in an oilseed rape flower is receptive to pollen whilst the flower is open. We assume that there is no diurnal variation in stigma reception during the daytime. Flowers open early in the morning (before the release of pollen begins), and close in the evening (after the release of pollen ends).
(d) Stigma reception: seasonal variation
The seasonal variation in stigma reception is a function of the proportion of flowers that are open, which is proportional to the rate of release of pollen from the field, i.e., a normal distribution with ![]() (stigma) = 7.8 days.
(stigma) = 7.8 days.
Maize (Zea mays)
Maize is a staple food for a significant proportion of the world’s population (Eastham and Sweet 2002). Furthermore, maize is grown as a livestock fodder crop in Europe and elsewhere (Eastham and Sweet 2002).
There is a medium to high risk of crop-to-crop gene flow in maize in the European Union (Eastham and Sweet 2002), partly due to the high cross-pollination rate (approximately 95%) (Ingram 2000).
Maize is almost completely wind pollinated. Most maize grown in the UK is a forage crop because the climate is not warm enough for the food varieties.
(a) Pollen release: diurnal variation (![]() )
)
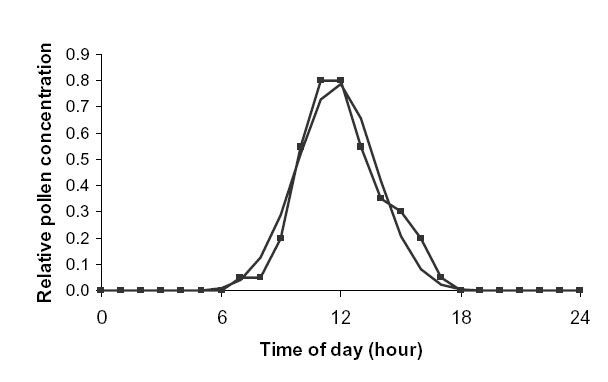
Jarosz et al. (2003) measured the diurnal variation in the concentration of pollen in the air from a source plot of maize over five days in the year 2000. Pollen emission began at about 08:00 and ended at about 16:00, with the maximum concentration (hmax) at approximately 12:00. The mean pollen concentration over the five days, given in Fig. 3(b) of Jarosz et al. (2003), was fitted to a normal distribution (Fig. D5). In this case, ![]() = 2.0 hours.
= 2.0 hours.
(b) Pollen release: seasonal variation (![]() )
)
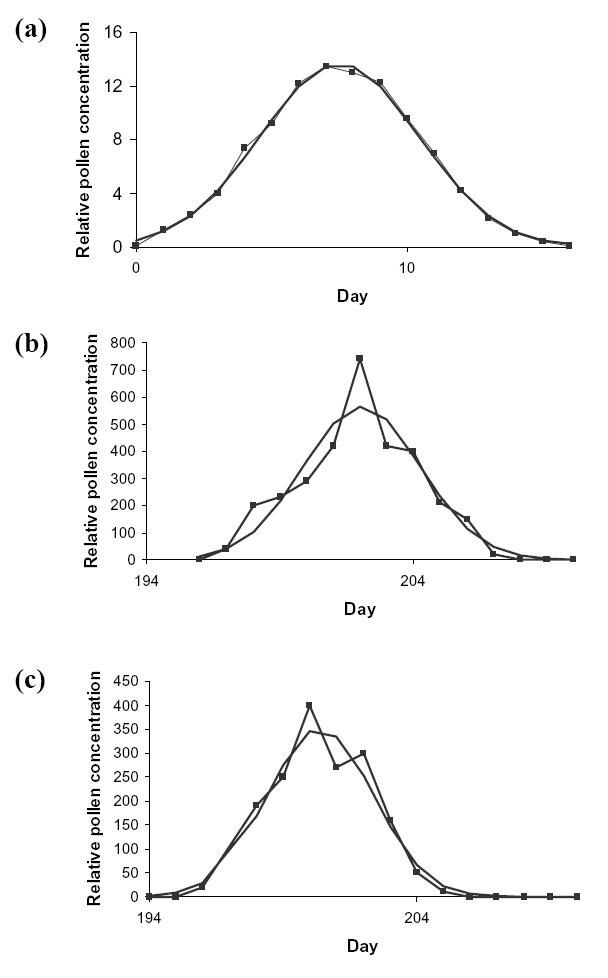
Table 3 in Jarosz et al. (2003) gives the number of pollen grains released for each of 17 days. In this case ![]() = 2.9 days (Fig. D6a).
= 2.9 days (Fig. D6a).
Figure 4 in Westgate et al. (2003) gives the seasonal variation in pollen release from maize plants of various degrees of male sterility. The level of male sterility was controlled either by detasselling or by a male sterile isoline. We fitted normal distributions to two datasets: to 100% male fertile, detasselled group and 75% male fertile, male sterile isoline group (Fig D6b,c). ![]() was found to be 2.2 and 2.0 days, respectively, with an average of 2.1 days.
was found to be 2.2 and 2.0 days, respectively, with an average of 2.1 days.
The average value of ![]() from these two studies is
from these two studies is ![]() = 2.5 days. Note that the flowering period of sweet corn in the UK is similar to that of maize (Ingram 2000).
= 2.5 days. Note that the flowering period of sweet corn in the UK is similar to that of maize (Ingram 2000).
Maize generally flowers in the last week of July and the first week of August (N. Jones, personal communication). Peak flowering (tmax) was set at 1st August.
(c) Stigma reception: diurnal variation
Maize stigmas and pollen are located on different structures of the plant. Stigmas are borne on silks and are receptive to pollen for about 14 days, which coincides with the main pollen release period (Emberlin et al. 1999). We assume that there is no change in stigma receptivity during the day.
(d) Stigma reception: seasonal variation
Silk emergence can start either during, after or before pollen shed, depending on growing conditions (M. Westgate, personal communication). Stigmas on a single plant are receptive to pollen for approximately the same period as pollen release of a single plant. Furthermore, the number of plants becoming receptive to pollen and releasing pollen are assumed to follow a normal distribution. Hence we assume that the number of receptive stigmas in a field also follows a normal distribution with ![]() (stigma) = 2.1 days. Additionally, any lag between silk emergence and pollen shed will not affect the expected relative cross-pollination rates (Eq. 4, main text).
(stigma) = 2.1 days. Additionally, any lag between silk emergence and pollen shed will not affect the expected relative cross-pollination rates (Eq. 4, main text).
Rice (Oryza rufipogon)
Rice is one of the most important crops in the world, providing staple food for over 50% of the world’s population (Song et al. 2003). Rice is not a major crop in Europe. However, we have included the crop in this study because the results from the European analysis will be suggestive of those found in other parts of the world, where rice is a major crop. A large range of genetically engineered traits has been introduced into rice (Messeguer et al. 2004). Although rice is autogamous, cross-pollination does occur, but only at very low levels (Messeguer et al. 2001).
Although the recommended isolation distance for rice is 10 m (Khush 1993), pollen has been measured at 110 m from the source (Song et al. 2003). However, it is generally acknowledged that gene flow between cultivated and wild rice occurs over a relatively short distance compared to crops such as maize, sugar beet, and oilseed rape (Song et al. 2003).
(a) Pollen release: diurnal variation (![]() )
)
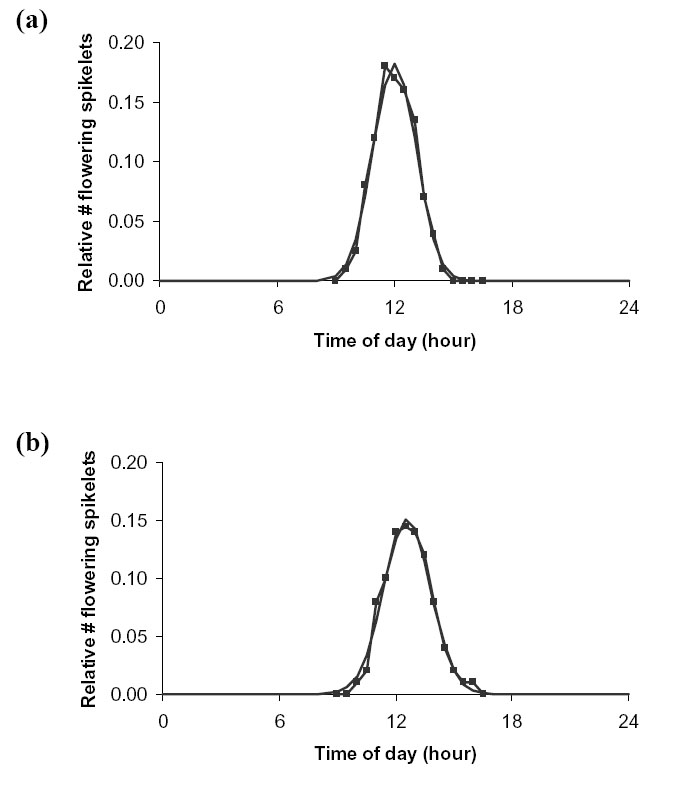
Song et al . (2003) found that the rice variety Minghui-63 flowered from 9:30–14:40 hours, and wild rice ( Oryza rufipogon) from 10:30 to 16:30. The exact diurnal flowering profile, based on the percentage of flowering rice spikelets (Z. Song, personal communication) (Fig. D7) gave ![]() = 1.1 hours for variety Minghui, with a peak at 12:00, and
= 1.1 hours for variety Minghui, with a peak at 12:00, and ![]() = 1.2 hours for wild rice, with a peak at 12:30.
= 1.2 hours for wild rice, with a peak at 12:30.
On average, ![]() = 1.1 hours, with the peak (hmax) at 12:00 hours. It should be noted that the timing of flowering depends to some extent on the variety, humidity and temperature (Grist 1953).
= 1.1 hours, with the peak (hmax) at 12:00 hours. It should be noted that the timing of flowering depends to some extent on the variety, humidity and temperature (Grist 1953).
(b) Pollen release: seasonal variation (![]() )
)
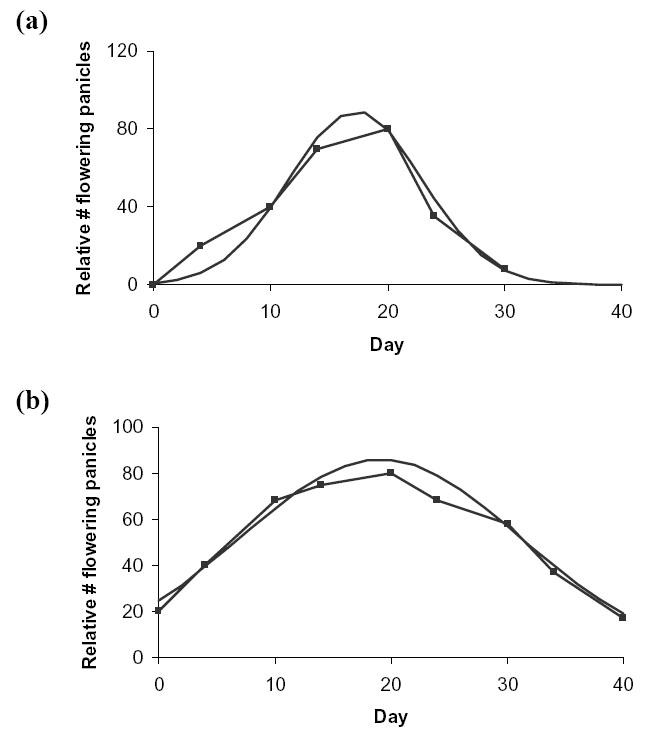
Unpublished data on the percentage of flowering rice panicles in a rice field from Z. Song gave ![]() as 5.7 days for the variety Minghui and 11.9 days for wild rice (Fig. D8).
as 5.7 days for the variety Minghui and 11.9 days for wild rice (Fig. D8).
J. Messeguer estimated ![]() as 2.8 days for the variety Senia, 3.0 days for Transgenic Senia, and 3.5 days for red rice (J. Messeguer, personal communication). The average of these figures is 3.1 days. In each case,
as 2.8 days for the variety Senia, 3.0 days for Transgenic Senia, and 3.5 days for red rice (J. Messeguer, personal communication). The average of these figures is 3.1 days. In each case, ![]() is calculated from the “flowering period” (period (± 2 SD) from the initiation of flowering until the last rice spike begins to flower) and the “opening period”, the time over which flowers are open on a single spike.
is calculated from the “flowering period” (period (± 2 SD) from the initiation of flowering until the last rice spike begins to flower) and the “opening period”, the time over which flowers are open on a single spike.
In summary, the flowering period of rice has been measured to vary widely, from ![]() = 2.8 to 11.9 days. For the purposes of our model, we took
= 2.8 to 11.9 days. For the purposes of our model, we took ![]() as the average over this range, i.e., 7.4 days.
as the average over this range, i.e., 7.4 days.
In Europe, rice flowers mainly between the last week of July and the first week of August depending on the variety and climatic conditions ( J. Messeguer, personal communication). Peak flowering (tmax) was set at 15th August.
(c) Stigma reception: diurnal variation
Rice stigmas and pollen are held in the same flowers. The period when flowers are open depends on the humidity, and may last from as little as six minutes to one hour per day (Grist 1953). For any given flower, the stigmas will be receptive when pollen is released into the air (when the flower is open). Hence we modeled the relative receptivity of the stigmas in a field as proportional to the relative concentration of pollen: a normal distribution with ![]() (stigma)= 1.1 hours, peaking (hmax) at 12:00 hours (Eq. 4, main text).
(stigma)= 1.1 hours, peaking (hmax) at 12:00 hours (Eq. 4, main text).
(d) Stigma reception: seasonal variation
Given that rice stigmas are receptive to pollen over the seasonal period when pollen is released, ![]() (stigma) = 7.4 days, the same as for pollen release.
(stigma) = 7.4 days, the same as for pollen release.
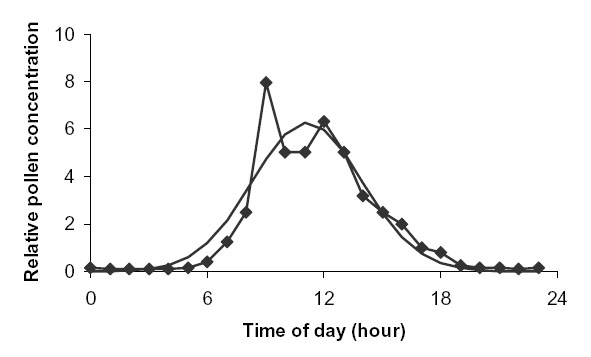
|
| FIG. D1. Actual (connected line) vs. fitted normal distribution (continuous line) diurnal variation in pollen air concentration for sugar beet. Data from Scott (1970). |
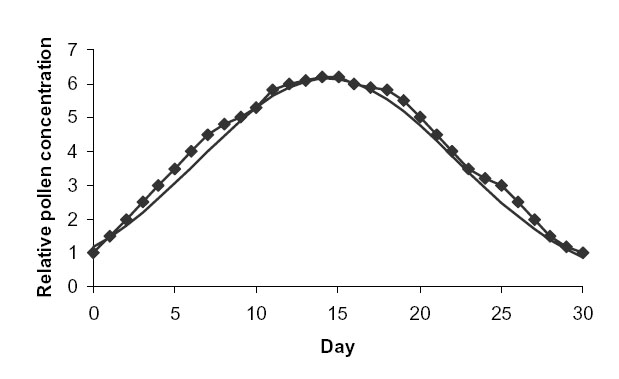
|
| FIG. D2. Actual (connected line) vs. fitted normal distribution (continuous line) seasonal variation in pollen air concentration for sugar beet. Data from Scott (1970). |
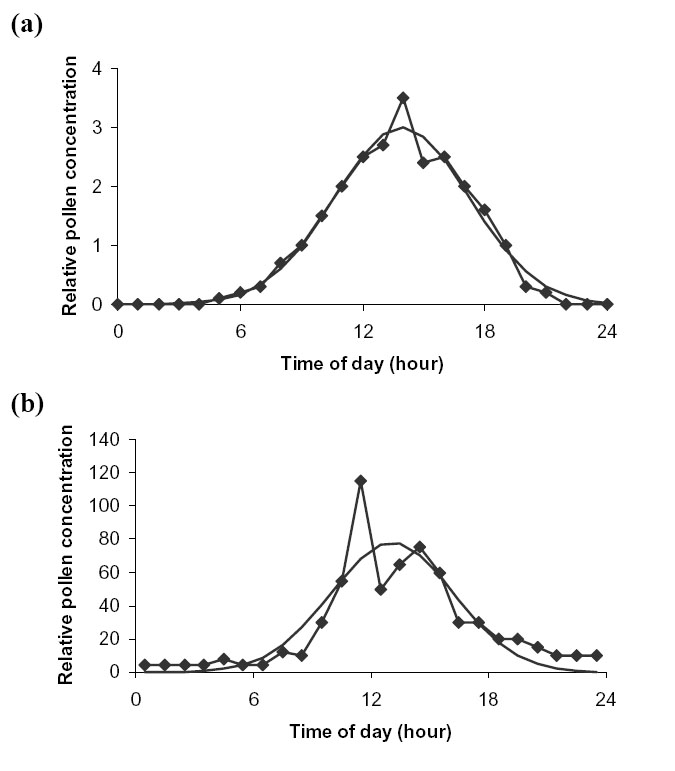
|
| FIG. D3. Average actual (connected line) vs. fitted normal distribution (continuous line) diurnal variation in pollen air concentration for oilseed rape. Data from (a) McCartney and Lacey (1991) and (b) Williams (1984). |
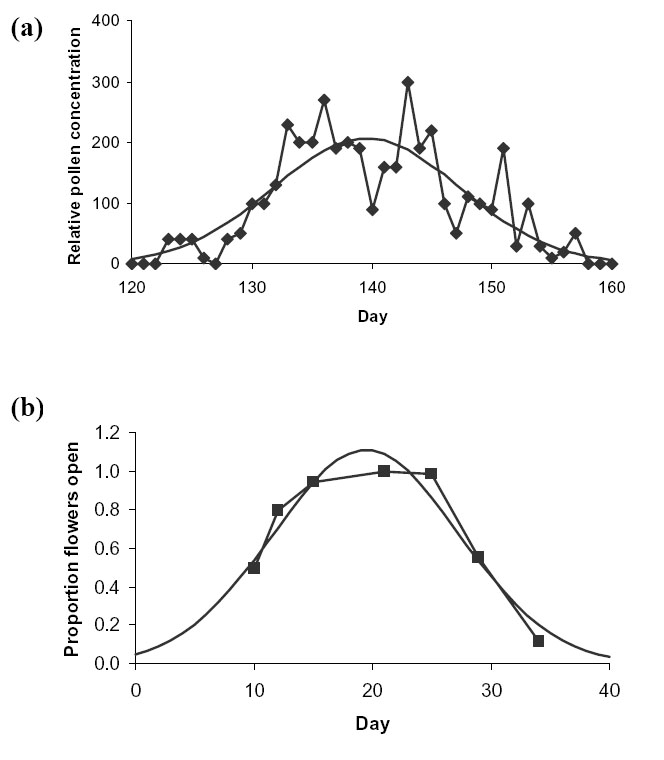
|
| FIG. D4. Average actual (connected line) vs. fitted normal distribution (continuous line) seasonal variation in (a) pollen air concentration for canola (from McCartney and Lacey 1991) and (b) proportion of flowers open (from Scheffler et al. 1995). |
|
| FIG. D5. Actual (connected line) vs. fitted normal distribution (continuous line) diurnal variation in pollen air concentration for maize. Data from Jarosz et al. (2003). |
|
| FIG. D6. Average actual (connected line) vs. fitted normal distribution (continuous line) seasonal variation in pollen air concentration for maize (a) from Jarosz et al. 2003, (b) 100% male fertile, detasselled plants, and (c) 75% male fertile, male sterile isoline plant from Westgate et al. (2003). |
|
| FIG. D7. Actual (connected line) vs. fitted normal distribution (continuous line) diurnal variation in relative number of flowering spikelets for (a) variety Minghui (b) wild rice. Data from Z. Song (personal communication). |
|
| FIG. D8. Actual (connected line) vs. fitted normal distribution (continuous line) seasonal variation in relative number of flowering rice panicles for (a) variety Minghui (b) wild rice. Data from Z. Song (unpublished). |
LITERATURE CITED
Eastham, K., and J. Sweet. 2002. Genetically modified organisms (GMOs): The significance of gene flow through pollen transfer. In Environ. Issue Rpt. No. 28. European Environment Agency, Copenhagen, Denmark.
Emberlin, J., B. Adams-Groom, and J. Tidmarsh. 1999. A report on the dispersal of maize pollen. Report for the Soil Association. <http://www.mindfully.org/GE/Dispersal-Maize-Pollen-UK.htm>
Grist, D. H. 1953. Rice. Longmans, Green & Co., London, UK.
Hayter, K. E., and J. E. Cresswell. 2006. The influence of pollinator abundance on the dynamics and efficiency of pollination in arable Brassica napus: implications for landscape-scale gene dispersal. Journal of Applied Ecology 43:1196–1202.
Ingram, J. 2000. Report on the separation distances required to ensure cross-pollination is below certain specified limits in non-seed crops of sugar beet, maize and oilseed rape. National Institute of Agricultural Botany, Cambridge, UK.
Jarosz, N., B. Loubet, B. Durand, A. McCartney, X. Foueillassar, and L. Huber. 2003. Field measurements of airborne concentration and deposition rate of maize pollen. Agricultural and Forest Meteorology 119:37–51.
Khush, G. S. 1993. Floral structure, pollination biology, breeding behaviour, transfer distance and isolation considerations. World Bank Technical Paper. Biotechnology Series No 1. Rice Biosafety. The Rockefeller Foundation, USA.
McCartney, H. A., and M. E. Lacey. 1991. Wind dispersal of pollen from crops of oilseed rape (Brassica napus L.). Journal of Aerosol Science 22:467–477.
McGregor, S. E. 1976. Insect Pollination Of Cultivated Crop Plants. < http://gears.tucson.ars.ag.gov/book>
Messeguer, J., C. Fogher, E. Guiderdoni, V. Marfà, M. M. Català, G. Baldi, and E. Melé. 2001. Field assessments of gene flow from transgenic to cultivated rice (Oryza sativa L.) using a herbicide resistance gene as tracer marker. Theoretical and Applied Genetics 103:1151–1159.
Messeguer, J., V. Marfà, M. M. Català, E. Guiderdoni, and E. Melé. 2004. A field study of pollen-mediated gene flow from Mediterranean GM rice to conventional rice and the red rice weed. Molecular Breeding 13:103–112.
Scheffler, J. A., R. Parkinson, and P. J. Dale. 1995. Evaluating the effectiveness of isolation distances for field plots of oilseed rape (Brassica napus) using a herbicide-resistance transgene as a selectable marker. Plant Breeding 114:317–21.
Scott, R. K. 1970. The effect of weather on the concentration of pollen within sugar-beet seed crops. Annals of Applied Biology 66:119–127.
Song, Z. P., B. Lu, Y. G. Zhu, and J. K. Chen. 2003. Gene flow from cultivated rice to the wild species Oryza rufipogon under experimental field conditions. New Phytologist 157:657–665.
Treu, R., and J. Emberlin. 2000. Pollen dispersal in the crops Maize (Zea mays), Oilseed rape (Brassica napus ssp. Oleifera), Potatoes (Solanum tuberosum), Sugar beet (Beta vulgaris ssp. vulgaris) and wheat (Triticum aestivum). Soil Association, Bristol, UK.
Westgate, M. E., J. Lizaso, and W. Batchelor. 2003. Quantitative relationships between pollen shed density and grain yield in maize. Crop Science 43:934–942.
Williams, I. H. 1984. The concentrations of air-borne rape pollen over a crop of oil-seed rape (Brassica napus L.). Journal of Agricultural Science 103:353–357.